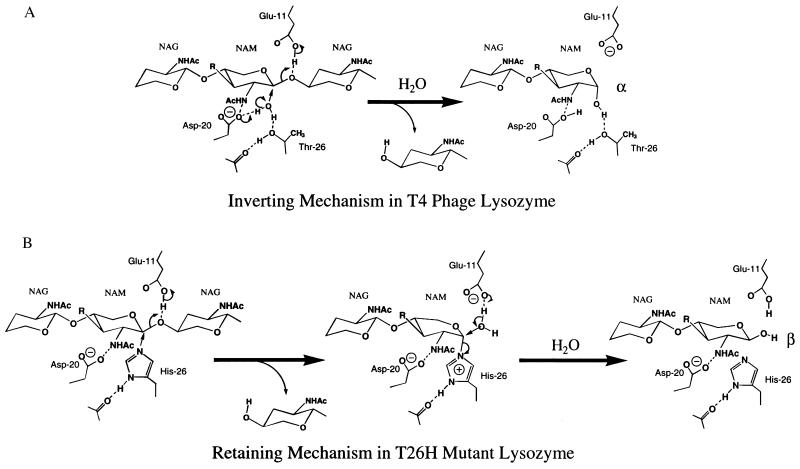
Figure 4.
Schematic diagram showing the overall relationship between the active-site structures and the presumed mechanisms of WT* T4L and mutants T26E and T26H. (A) In WT* lysozyme the water molecule hydrogen-bonded to Asp-20 and Thr-26 is presumed to act as the nucleophile, attacking the C-1 carbon of NAM. (B) In mutant T26H the nitrogen of His-26 occupies a position close to the water molecule in WT* and is presumed to act as the nucleophile, leading to a covalent adduct. This adduct can either break down by the addition of water, as shown, or can be subject to attack by another disaccharide, leading to transglycosylation. In T26E (not shown) an oxygen of Glu-26 occupies a position close to the water molecule and attacks the C-1 carbon to yield a stable enzyme-substrate adduct (13).