Abstract
Aim: To evaluate the benefits of macular translocation with 360 degree retinotomy in patients with exudative age related macular degeneration (ARMD).
Methods: A consecutive interventional case series was performed on patients who underwent macular translocation between June 1997 and January 2000 at the department of ophthalmology, University of Aachen, Germany. A retrospective pilot study was set up with a minimum follow up of 12 months in 39 consecutive patients with subfoveal choroidal neovascularisation secondary to ARMD. The surgical technique included pars plana vitrectomy, induction of retinal detachment, 360 degree retinotomy, removal of the choroidal neovascular membranes (CNVM), macular translocation, peripheral laser retinopexy, and silicone oil endotamponade.
Results: 18 patients showed predominantly occult CNVM, six patients had predominantly classic CNVM, and 15 showed subretinal haemorrhage. At the 12 month follow up 13 patients (33%) showed an improvement in visual acuity of more than three lines (logMAR scale), 18 patients (46%) retained stable visual acuity with a change of equal or less than three lines (logMAR scale), and eight patients (21%) showed a decrease in visual acuity of more than three lines (logMAR scale). Recurrence of CNVM was observed in three (8%) eyes at 5–11 months postoperatively. Other complications included proliferative vitreoretinopathy with retinal detachment (n=10), peripheral epiretinal membranes (n=9), macular pucker (n=2), corneal decompensation (n=2), and hypotony (n=11). 18 patients (46%) complained about persistent diplopia.
Conclusion: Macular translocation surgery is able to maintain or improve distant vision in the majority of patients with exudative ARMD. Proliferative vitreoretinopathy and diplopia are the two major complications. A prospective randomised controlled trial comparing macular translocation with observation for patients with the occult form of exudative ARMD may be justified.
Keywords: age related macular degeneration, choriodal neovascular membrane, macular translocation
Exudative age related macular degeneration (ARMD) is the major cause of legal blindness in the elderly population. 1 It is in part characterised by the growth of new blood vessels from the choroid into the subretinal or subpigment epithelial space where it ultimately leads to scar formation of retinal pigment epithelium (RPE) and degeneration of retinal photoreceptors. Surgical extraction of CNVM alone is unsatisfactory in view of visual rehabilitation since the RPE is being removed or severely damaged.2–4 The rationale of macular translocation is based on the hypothesis that ARMD begins in the layer of RPE and that the diseased RPE is more or less confined to the area of the macula. Macular translocation aims to bring the fovea onto functional or at least less diseased RPE.5,6 Macular translocation with 360 degree retinotomy is accompanied by significant risks, such as PVR and diplopia. In addition, these older patients must endure at least two surgical interventions (macular translocation, silicone oil removal with extraocular muscle surgery). Earlier reports with short term follow up suggest that macular translocation results in visual stabilisation or even in improvement of visual acuity in some patients.7–9 We report a long term (1 year) follow up on 39 patients to investigate visual prognosis and complications of macular translocation.
PATIENTS AND METHODS
Macular translocation surgery was performed in 39 eyes of 39 patients (22 female, 17 male) aged 60–87 years (mean 74.2 (SD 5.9) years, Table 1), between June 1997 and January 2000 at the department of ophthalmology, University of Aachen, Germany. Patients had to meet the following inclusion criteria:
aged 60 years or older
ocular refraction less than −6 dioptres
presence of subretinal neovascular membranes secondary to ARMD involving the geometric centre of the foveal avascular zone,10 or
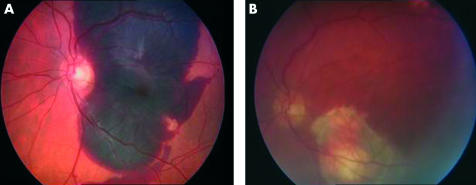
presence of thick subfoveal haemorrhage11,12 secondary to CNVM due to ARMD (Fig 1)
presence of other clinical signs of ARMD such as soft drusen, retinal pigment epithelial changes, or disciform scars
loss of ability to read Birkhäuser charts in the eye to be operated on
follow up of a minimum of 12 months.
Table 1.
Demographic data of patients included and their distribution according to subgroups
| Sex | |||||||
| Group | No | Mean age (years) | M | F | Duration of visual impairment (weeks) | Last eye* | Degree of rotation |
| Occult CNVM | 18 | 64–81 | 9 | 9 | 2–52 | 10 | 10–45 |
| 74.4 | |||||||
| Classic CNVM | 6 | 66–83 | 2 | 4 | 8–52 | 4 | 30–60 |
| 75.7 | |||||||
| Subretinal haemorrhage | 15 | 60–87 | 6 | 9 | 1–16 | 12 | 20–55 |
| 73.5 | |||||||
| Total | 39 | 60–87 | 17 | 22 | 1–52 | 26 | 10–60 |
| 74.2 (5.9) | |||||||
*The operated eye was the better eye at time of operation.
Figure 1.
Subfoveal haemorrhage, (A) preoperative, (B) postoperative with scar at the original site of the fovea.
Exclusion criteria:
other ocular pathology or previous surgery, except for IOL implantation in the affected eye.
Patients were classified by fluorescein angiography into three groups. Patients who showed classic or predominantly classic membranes and patients who showed occult or predominantly occult membranes (according to the Macular Photocoagulation Study)13 (see Fig 3). The area of the membrane was measured with the aid of the scanning laser ophthalmoscope (Heidelberg Germany) by marking the edge of the different components; the instrument calculates the different areas.
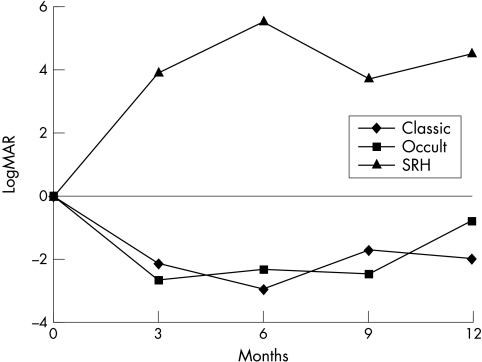
Figure 3.
Visual acuity over time.
A third group included patients with massive subfoveal haemorrhage.11,12 Blood was an indication to translocation surgery, when thick haemorrhage covered the entire posterior pole of the eye and beyond the temporal vascular arcade and thus was judged to require a large retinotomy to evacuate blood clots Those cases were not expected to improve on injection of rTPA and gas alone11,12 (Fig 2).
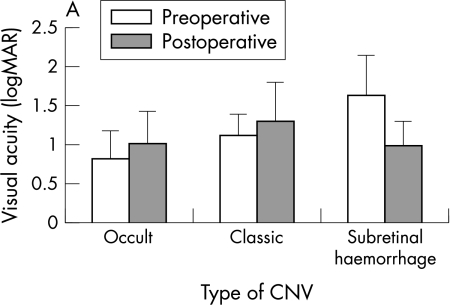
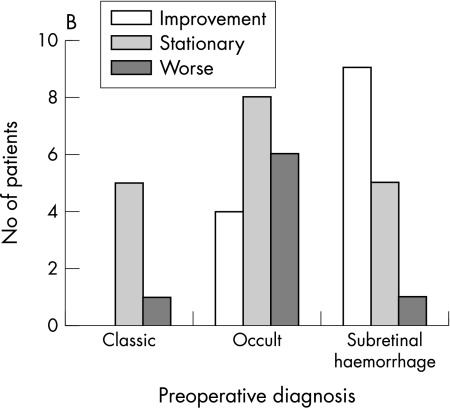
Figure 2.
(A) Mean preoperative and postoperative visual acuity after macular translocation. (B) Visual prognosis in different subgroups: improvement or deterioration means change of three lines logMAR units. (C) Preoperative and postoperative visual acuity after macular translocation.
Informed consent was obtained from each patient, indicating that the procedure was a novel experimental approach and an alternative to observation, conventional membrane extraction surgery, laser photocoagulation, and radiation therapy. Photodynamic therapy was not yet approved at that time in Germany.
Ophthalmological examination
Visual acuity, slit lamp microscopy, ophthalmoscopy, intraocular pressure, and orthoptic status were assessed preoperatively, 3 weeks, 3 months, 6 months, and 12 months following surgery and every 3 months thereafter up to the last visit of follow up. Fundus photography, fluorescein and indocyanin green angiography were performed preoperatively and at irregular intervals thereafter (for example, to assess CNVM recurrence).
Visual acuity
Visual acuity was determined with refraction using the Snellen visual acuity (VA) chart at 5 metres. In patients not seeing the largest letters at this distance (5/50) the chart was brought closer to the patient, the nominator of the Snellen fraction was changed accordingly (4/50, 3/50, etc). Counting fingers was performed at a distance of 50 cm, hand movement and perception of light were tested at 30 cm. For statistical analysis the Snellen measurements were converted to the logMAR scale.14 A significant change in VA was defined as a gain or loss of three lines or more in the logMAR scale (increase of three lines of logMAR scale equals doubling of the visual angle).14 A change of VA from counting fingers (CF) to hand movement (HM) and from HM to light perception (LP) was classified as a significant change in the VA. CF was assigned to 1/50 on Snellen chart, or 1.7 on the logMAR scale on the assumption that it cannot be better than seeing the largest letter on the chart offered at 1 metre. Accordingly, HM was assigned to 2 on the logMAR scale, and LP was assigned to 2.3 on the logMAR scale. No patient had reading vision on the scale of “Birkhäuser” reading charts preoperatively, since loss of reading vision (Birkhäuser reading charts) was our precondition to perform translocation surgery.
Orthoptic examination
Orthoptic examination included cover test, Bagolini’s striated glasses test, monocular measurement of cyclorotation with Maddox rod cylinder, testing of ocular motility and, if possible, the measurement of the subjective squint angle with a light red glass.
Surgical technique
Lens
In the first 12 patients the lens was removed either at the time of the macular translocation (n=9) or at the time of silicone removal (n=3) using phacoemulsification. In the other 27 patients, phakic eyes underwent phacoemusification and posterior chamber lens implantation 10 days before macular translocation surgery.
Macular translocation
Macular translocation surgery was performed under general anaesthesia. The first 17 patients received an encircling band (2.5 mm). All patients underwent a three port pars plana vitrectomy (ppV). Retinal detachment was initiated by the induction of a small retinal bleb obtained by injecting into the subretinal space a stream of balanced salt solution (BSS) with a 40 gauge needle through a small retinotomy. Retinal detachment was completed by injecting BSS through the sclera under the detached area with a 21 gauge needle. The 360 degree retinotomy was performed with the vitreous cutter at the ora serrata. The neovascular membrane was dissected from the choroid and removed. The blood vessel stalk was coagulated by bipolar diathermy. The central retina was stabilised by injection of about 1.0 ml of liquid semifluorinated alcane (perfluorohexyloctane). The retina was rotated around the optic nerve head so that the fovea was located 0.5–1.0 disc diameter outside of the edge of the RPE defect.
The decision to perform superior or inferior macular translocation15 was based on preoperative angiographic findings as well as on retinal pigment epithelial (RPE) biomicroscopic findings. Thirty one patients had a superior translocation of the macula (10 without counter-rotation and 21 with counter-rotation) and eight patients had an inferior translocation of the macula (two without counter-rotation and six with counter-rotation). The retina was then hydraulically unfolded by the additional injection of liquid perfluorocarbon. Laser retinopexy was performed at the border of the 360 degree retinotomy. Finally, liquid perfluorocarbon was exchanged against a 5000 cSt silicone oil endotamponade. The silicone oil was removed 3 months later.
Counter-rotation
In the first 12 patients no counter-rotation was done. Counter-rotation of the globe was performed in the following 27 patients either at the time of macular translocation (n=16) or in the following 11 eyes at the time of silicone oil removal.
Excyclorotation of the globe (following an upward rotation of the macula) included surgery of all six eye muscles. The anterior four fifths of the superior oblique muscle were recessed 14 mm, combined with a small posterior transposition. The anterior part of the inferior oblique muscle was folded 7 mm and the fold was advanced another 7 mm. Subsequently, a transposition of parts of all rectus muscles was performed. The temporal portion of the superior rectus (one quarter of the muscle) was disinserted, pulled over the remaining portion, and fixed at the upper margin of the medial rectus. Similarly, the upper portion of the medial rectus was transposed to the medial margin of the inferior rectus. Corresponding procedures were performed on the inferior and lateral rectus muscles. The fascicles of the rectus muscles were pulled over the remaining muscle to avoid scarring between them and the operated oblique muscles.
Incyclorotation of the globe (following downward rotation of the macula) was achieved by transposition of parts of all rectus muscles following a reverse procedure as outlined above. In addition, the inferior oblique muscle was recessed 11 mm and the anterior part of the superior oblique was folded 12 mm.
RESULTS
All patients had a history of loss of reading vision, with blurred vision or metamorphopsia in the operated eye for 1–52 weeks (mean 14 weeks) (Table 1). Follow up ranged from 12 months to 2.3 years (mean 1.3 (SD 0.3) years). In 26 patients the operated eye had a better visual acuity than the non-operated eye. Eighteen eyes showed an occult or predominantly occult CNVM, six eyes showed a classic or predominantly classic CNVM, and 15 eyes presented with subretinal haemorrhage.
Visual acuity
The initial best corrected VA ranged from 20/60 to light perception (mean 20/260). At 12 months after macular translocation the VA ranged from 20/40 to hand movement (mean 20/260) (Tables 2 and 3). Initial and 1 year visual acuity were not significantly changed. (Mann-Whitney U test, p = 0.9344). Thirteen patients showed improvement of visual acuity of more than three lines (logMAR scale), 18 patients retained stable visual acuity with a change of equal or less than three lines (logMAR scale), and eight patients showed decreased visual acuity of more than three lines (logMAR scale) (Table 2, Fig 2). Significant loss of visual acuity could be explained in five out of the eight eyes by intraoperative or postoperative complications. Patients with classic CNVM (p = 0.8302) and patients with occult CNVM (p = 0.1251) showed no significant change in visual acuity. Visual acuity improved significantly in patients with subretinal haemorrhage (p = 0.0122, two tailed).
Table 2.
Original data of all patients
| Preoperative visual acuity | Postoperative visual acuity | |||||||||
| No | Initial | Age (years) | Sex | Duration of visual impairment | Last eye | Type of CNV | Deci | Log MAR | Deci | Log MAR |
| 1 | BW | 70 | M | 3 wk | N | occult | 0.16 | 0.8 | 0.125 | 0.9 |
| 2 | BL | 81 | M | 16 wk | N | occult | 0.05 | 1.3 | 0.033 | 1.5 |
| 3 | EL | 64 | F | 12 wk | N | occult | 0.2 | 0.7 | 0.2 | 0.7 |
| 4 | HA | 75 | F | 20 wk | J | occult | 0.05 | 1.3 | 0.1 | 1.0 |
| 5 | HH | 67 | M | 24 wk | N | occult | 0.4 | 0.4 | 0.06 | 1.2 |
| 6 | HR | 78 | F | 24 wk | N | occult | 0.1 | 1.0 | 0.2 | 0.7 |
| 7 | KK | 72 | F | 2 wk | J | occult | 0.3 | 0.5 | 0.1 | 1.0 |
| 8 | KE | 75 | F | 2 wk | N | occult | 0.20 | 0.7 | 0.04 | 1.4 |
| 9 | KG | 76 | M | 2 wk | N | occult | 0.4 | 0.4 | 0.05 | 1.3 |
| 10 | LI | 71 | F | 3 wk | J | occult | 0.03 | 1.5 | 0.02 | 1.7 |
| 11 | MG | 67 | M | 5 wk | N | occult | 0.16 | 0.8 | 0.6 | 0.2 |
| 12 | MR | 81 | F | 52 wk | J | occult | 0.1 | 1.0 | 0.08 | 1.1 |
| 13 | MB | 76 | M | 12 wk | J | occult | 0.1 | 1.0 | 0.1 | 1.0 |
| 14 | PE | 73 | M | 3 wk | N | occult | 0.16 | 0.8 | 0.4 | 0.4 |
| 15 | SK | 73 | M | 8 wk | J | occult | 0.3 | 0.5 | 0.05 | 1.3 |
| 16 | SM | 75 | F | 12 wk | J | occult | 0.3 | 0.5 | 0.1 | 1.0 |
| 17 | TA | 76 | M | 4 wk | J | occult | 0.32 | 0.5 | 0.32 | 0.5 |
| 18 | VG | 78 | M | 52 wk | J | occult | 0.05 | 1.3 | 0.03 | 1.5 |
| 19 | NE | 70 | F | 4 days | N | SRH | 0.01 | 2.0 | 0.028 | 1.6 |
| 20 | HA | 75 | F | 4 days | J | SRH | 0.02 | 1.7 | 0.1 | 1.0 |
| 21 | DP | 84 | M | 10 days | J | SRH | 0.01 | 2.0 | 0.1 | 1.0 |
| 22 | MM | 78 | F | 5 wk | J | SRH | 0.01 | 2.0 | 0.066 | 1.2 |
| 23 | FH | 74 | M | 4 wk | J | SRH | 0.005 | 2.3 | 0.06 | 1.2 |
| 24 | AM | 70 | M | 24 wk | N | SRH | 0.02 | 1.7 | 0.04 | 1.4 |
| 25 | UF | 68 | M | 2 wk | J | SRH | 0.04 | 1.4 | 0.117 | 0.9 |
| 26 | SM | 60 | F | 20 wk | J | SRH | 0.1 | 1.0 | 0.03 | 1.5 |
| 27 | LA1 | 79 | F | 5 days | J | SRH | 0.025 | 1.6 | 0.05 | 1.3 |
| 28 | LA2 | 68 | F | 8 wk | N | SRH | 0.25 | 0.6 | 0.1 | 1.0 |
| 29 | SH | 62 | M | 12 wk | N | SRH | 0.01 | 2.0 | 0.01 | 2.0 |
| 30 | KE | 78 | F | 24 wk | J | SRH | 0.01 | 2.0 | 0.033 | 1.5 |
| 31 | VA | 79 | F | 16 wk | J | SRH | 0.03 | 1.5 | 0.02 | 1.7 |
| 32 | KA | 87 | F | 1 wk | J | SRH | 0.01 | 2.0 | 0.066 | 1.2 |
| 33 | HH | 71 | M | 8 wk | J | SRH | 0.16 | 0.8 | 0.1 | 1.0 |
| 34 | TK | 81 | M | 40 wk | J | classic | 0.125 | 0.9 | 0.01 | 2.0 |
| 35 | DA | 76 | F | 52 wk | J | classic | 0.05 | 1.3 | 0.04 | 1.4 |
| 36 | KA | 83 | F | 16 wk | J | classic | 0.125 | 0.9 | 0.2 | 0.7 |
| 37 | SM | 73 | F | 12 wk | J | classic | 0.066 | 1.2 | 0.08 | 1.1 |
| 38 | PI | 73 | F | 8 wk | N | classic | 0.03 | 1.5 | 0.02 | 1.7 |
| 39 | SR | 66 | M | 24 wk | N | classic | 0.125 | 0.9 | 0.125 | 0.9 |
Y = yes; N = no; wk = weeks; D = days.
Table 3.
Preoperative visual acuity and visual acuity change at 12 months in the different subgroups
| Visual acuity | VA change at 12 months‡ | |||||
| Group | Preoperative | At 12 months | Improvement | Stationary | Loss | Av of lines gained or lost at 12 months |
| Occult CNV | 20/60 to 20/600 (0.39 to 1.52)* (0.83 (0.35))† | 20/40 to CF (0.22 to 1.7)* (1.02 (0.41))† | 4 | 8 | 6 | −0.81 |
| Classic CNV | 20/200 to 20/600 (1 to 1.52)* (1.12 (0.26))† | 20/100 to HM (0.7 to 2)* (1.3 (0.5))† | – | 5 | 1 | −1.94 |
| Subretinal haemorrhage | 20/125 to LP (0.8 to 2.3)* (1.65 (0.47))† | 20/160 to HM (0.9 to 2)* (1 (0.31))† | 9 | 5 | 1 | +4.5 |
| Total | 20/60 to LP (0.39 to 2.3)* (1.19 (0.54))† | 20/40 to HM (0.22 to 2)* (1.17 (0.4))† | 13 | 18 | 8 | |
VA = visual acuity, Av = average, best corrected visual acuity, *logMAR scale; †Mean; ‡change equals gain or loss more than three lines on logMAR scale.
Anatomical outcome
Twelve months after macular translocation as well as at the last postoperative follow up, the retinas of all patients were attached. Translocation of the macula was effective in all but one eye where the fovea came to lie on the edge of intact RPE. The degree of rotation ranged from 10 degrees to 60 degrees. We observed three eyes with recurrent CNVM but no CNVM persistence.16 CNVM recurrence occurred only in conjunction with occult CNVM, from the site of intact RPE closest to the fovea, and at 5, 8, and 11 months postoperatively. Recurrence was addressed by argon laser coagulation.
Complications
Intraoperative complications included failure to achieve complete hydraulic retinal detachment in 15 patients (38.5%). In those eyes retinal detachment was completed by flute needle or forceps manoeuvres. Two patients developed a macular hole during hydraulic retinal detachment. Unintentional posterior retinal breaks occurred in one patient. In eight patients the neovascular membrane was adherent to the neurosensory retina (20.5%). Dissection of retina and CNVM caused retinal breaks in two patients (papillomacular bundle, n = 1; macula, n = 1). Severe bleeding with removal of the CNVM occurred in 12 cases (30.8%). Homeostasis was achieved by diathermy to the choroidal bleeding site and by raising the intraocular pressure.
Postoperative complications
Proliferative vitreoretinopathy (PVR) leading to retinal detachment occurred in 10 eyes (25.6%). Long term retinal reattachment was then achieved by one re-operation. In an additional nine eyes (23%) epiretinal membranes were “peeled” at the time of silicone oil removal from attached retina. Isolated macular pucker was encountered in two eyes. Corneal decompensation was observed in two eyes possibly due to silicone oil in the anterior chamber in one eye and secondary glaucoma in the other.
Hypotony (intraocular pressures less than 8 mm Hg) occurred in 11 eyes, two of which required silicone oil refill to prevent phthisis. Normal intraocular pressure (8–20 mm Hg) was measured in 26 eyes and elevated IOP (above 20 mm Hg) was observed in two patients.
Sensory outcome (Table 4)
Table 4.
Orthoptic result
| Patients | Without muscle surgery | With muscle surgery | ||||
| Total | 12 | 27*** | ||||
| Subjective diplopia | No | Rare | Disturbing | No | Rare | Disturbing |
| 5 | 1 | 7 | 5 | |||
| 42% | 8% | 26% | 18% | |||
| 6 | 6 | 15 | 12 | |||
| 50%** | 50% | 56% | 44% | |||
| Remaining cyclotropia | 25.3°* | 23.3°* | 7.1°* | 8.3°* | ||
| 14.2°† | 11.7°† | 8.9°† | 8.0°† | |||
| 2°–45° | 10°–40° | 0–25° | 0–25° | |||
| Difference in visual acuity between both eyes (in lines) | 5.5* | 5.5* | 4.6* | 5.3* | ||
| 3.9† | 2.9† | 3.0† | 2.4† | |||
| 1–11 | (2–10) | 0–10 | 2–11 | |||
| R*: 4 | R*: 5 | R*: 6 | R*: 6 | |||
| F*: 2 | F*: 1 | F*: 8 | F*: 6 | |||
| E*: 1 | ||||||
| Bagolini test | ||||||
| Positive | 0 | 0 | 7 | 1 | ||
| Exclusion rotated eye | 3 | 1 | 5 | 6 | ||
| Exclusion fellow eye | 2 | 3 | 2 | 3 | ||
| Diplopia | 0 | 0 | 0 | 1 | ||
| Not recognisable | 1 | 2 | 1 | 1 | ||
*Mean; †SD; ‡R = number of patients with better visual acuity of the rotated eye; F = number of patients with better visual acuity of the fellow eye; E = number of patients with equal visual acuity of both eyes.
**Including two patients without light perception of the fellow eye. If those two patients were excluded because they could not have double vision, 4 (40%) patients of the group with no muscle surgery had no diplopia and 6 (60%) had diplopia.
***In the first 16 patients counterrotation together with macula translocation, in the following 11 patients counterrotation together with silicon-oil removal.
12 patients without muscle surgery
All patients indicated a negative Bagolini’s striated glasses test, four out of 12 suppressed the rotated eye, and five patients suppressed the fellow eye. Three patients were not able to recognise the Bagolini’s striated glasses test. Six patients (50%) had no diplopia. Five patients (42%) perceived rare or hardly disturbing diplopia. Only one patient complained of disturbing diplopia. “Diplopia” was noted despite suppression of one eye in the Bagolini striated glasses test. Out of six patients who complained of “diplopia” it came down to “metamorphopsia” in the rotated eye (one patient) and monocular double vision in the rotated eye (two patients). We found no statistically significant difference in the degree of remaining cyclotropia comparing the patients with and without diplopia (unpaired Wilcoxon test = Mann-Whitney U test). The six patients with no diplopia had a remaining cyclotropia between 2° and 45°, mean 25.3° (SD 14.2°). Cerebral compensation of cyclorotation under monocular viewing conditions was enormous in one case: cyclotropia of 2° (Maddox rod) with “no light perception” in the other eye was coupled to an objective cyclorotation of 40°, as measured by ophthalmoscopy. The six patients with diplopia had a residual cyclotropia ranging between 10° and 40°, mean 23.3° (SD 11.7°). Residual cyclotropia was often affiliated with head tilting. Visual acuity was not significantly different between patients with and without diplopia. The mean difference in visual acuity between the treated and untreated eyes was 5.5 (SD 3.9) lines (logMAR scale) in the patients without diplopia and 5.5 (2.9) lines (logMAR scale) in the patients with diplopia. Four out of six patients without diplopia and five out of six patients with diplopia had a better visual acuity in the rotated eye.
27 patients with muscle surgery
With counter-rotation of the globe eight out of 27 patients (30%) had a positive Bagolini’s striated glasses test. Two patients were not able to recognise the Bagolini’s striated glasses test and one patient noted diplopia when tested with striated glasses. Eleven patients (41%) suppressed the rotated eye and five patients suppressed the fellow eye. Fifteen patients (56%) had no diplopia, seven patients (26%) had rare or hardly disturbing diplopia, and five patients (18%) had disturbing diplopia. Some patients complained of diplopia despite a positive Bagolini’s striated glasses test or despite suppression of one eye in the Bagolini’s striated glasses test. In those cases we noted aniseiconia with a smaller image of the rotated eye (three patients), vertical diplopia which could be corrected with prisms (two patients), or we found confusion (one patient).
There is no statistically significant difference in the degree of remaining cyclotropia comparing the patients with and without diplopia (U test). The 15 patients with no diplopia had a residual cyclotropia between 0° and 25°, mean 7.1° (8.9°). Six of those patients had a residual cyclotropia of 0°. The 12 patients with diplopia had a residual cyclotropia between 0° and 25°, mean 8.3° (8.0°). Three of those patients had a remaining cyclotropia of 0°. Some patients could not tolerate a little cyclotropia, whereas others had no problem in compensating a residual cyclotropia of 25°. The reduction of cyclotropia by counter-rotation ranged between 17° and 56° (mean 35° (SD 9.7°)). No patient was overcorrected. Five patients (19%) required additional surgery to compensate for residual cyclotropia or vertical diplopia. Visual acuity was not significantly different between patients with and without diplopia. The mean difference in visual acuity between both eyes was 4.6 (3.0) lines (logMAR scale) in the patients without diplopia and 5.3 (2.4) lines (logMAR scale) in the patients with diplopia. Six out of 15 patients without diplopia and six out of 12 patients with diplopia had a better visual acuity in the rotated eye.
There was no statistically significant difference in the number of patients suffering from diplopia comparing eyes without counter-rotation to eyes with counter-rotation. There is also no relation between the amount of remaining cyclotropia and subjectively felt diplopia. Lack of spatial orientation which implicates misjudgment of the three dimensional location of objects and improper visual hand coordination is a severe complaint, especially when the fellow eye is suppressed.
DISCUSSION
Subfoveal CNVM in age related macular degeneration generally leads to irreversible central visual loss. Only a minority of patients so far could be satisfactorily treated by thermal photocoagulation,17 photodynamic therapy,18 or surgical membrane extraction with and without pigment cell transplantation.19,20 Earlier studies on macular translocation showed stabilisation or improvement of visual acuity in two thirds of eyes9,21–23 with reading abilities improving more than distant visual acuity. We report, accordingly, improvement of distant visual acuity in 13 patients (33%), stabilisation in 18 patients (46%), and decrease in distant visual acuity in eight patients (21%). The best postoperative visual acuity was reached at 10–12 months after macular translocation surgery. This interval corresponded to 4–6 months after silicone oil removal. At the time of the last follow up there was no further significant change of visual acuity (12 months to 2.3 years postoperative, mean 16 (SD 4) months). Visual outcome suggests an advantage of macular translocation for eyes with occult CNVM, when compared to untreated eyes in the RAD study.24 The RAD study reports a loss of visual acuity of 3.3 (3.8) lines (logMAR scale) over 1 year in control eyes with occult CNVM. In our series treated eyes lost 0.81 (3.6) lines (logMAR scale) of visual acuity over the same period (Fig 3). Eyes with classic or predominant classic CNVM lost −1.94 (1.11) lines (logMAR scale) of visual acuity. There is no comparable series in the literature, since the size of the classic CNVMs in our series (2.5–7 disc areas) is larger than in the MPS study.13,17
Untreated eyes with submacular haemorrhage lose 1.25 lines (logMAR scale) of visual acuity over 1 year.11 Macular translocation provides a gain of the VA of + 4.5 (3.9) lines (logMAR scale) in our series (Fig 3). The advantage of macular translocation may be explained by the shorter interval between bleeding and surgical intervention (average 6 weeks) compared to eyes with occult or classic CNVM (occult CNVM average 9 weeks, classic CNVM 21 weeks).
We consider macular translocation as an experimental procedure with risk of visual loss. We therefore excluded patients with reading ability, which then precluded evaluation of reading vision. Accordingly, the preoperative visual acuity of our patients was considerably lower. Duration of loss of reading ability to the time of surgery ranged between 1–52 weeks (mean 14 weeks). Both may explain the low final visual acuity compared to others.9
Lack of improvement of visual acuity or loss of visual acuity can also in part be attributed to intraoperative and postoperative complications like PVR retinal detachment, macular pucker, macular hole, papillomacular bundle tear, and CNVM recurrence.
Our data suggest that the risk of PVR in macular translocation depends to some extent on learning a new surgical technique. In 199722 we reported a PVR incidence of 43%. We currently note an incidence of 20% in the last 30 eyes of this series. Accordingly, earlier communications note a rate of PVR of 60%.7,25 “Learning” consisted, for example, in avoiding retinotomies in the area just outside the vascular arcade. PVR membranes often developed from those retinotomy sites. We currently begin the hydraulic retinal detachment by parapapillary transretinal BSS injection (DeJuan cannula) and continue and complete retinal detachment by a transscleral BSS injection into the formed subretinal space.
We found a low rate of recurrence of CNVM, only three patients (8%) between 5 and 11 months postoperatively by means of ophthalmoscopy. The Macular Photocoagulation Study Group15 reports a rate of CNVM recurrence of 20%, and a series of surgically excised subfoveal CNVM report a recurrence rate of 24%.26 We did not regularly perform postoperative angiography, only when there were ophthalmoscopic macular changes suggestive of CNVM or macular oedema. We cannot exclude the fact that we would had found a higher rate of CNVM recurrence if angiography had been performed on a regular basis at every follow up. For the same reason we are unable to name the risk of chronic macular oedema, which is stated to be as high as 13% by others.9 Our rate of CNVM recurrence is in accordance with other series of macular translocation.9 The low rate of recurrence of CNVM following macular translocation may be a function of CNVM size. In our series the postoperative area of RPE defect after removal of the CNVM ranged from 2 to 9 disc areas (DA) (assuming 1 DA is equal to 1.77 mm2)13 with a mean of 4.9 (SD 1.9) DA. If the risk of CNVM recurrence is attributed to the dense network of subfoveal choriocapillaris, then removal of most of this vascular network together with large CNVMs may explain the low risk of recurrence. An advantage of macular translocation with 360° retinotomy over limited macular translocation8 is the freedom to rotate the fovea well over the edge into the area of intact RPE. We aim to gain a distance of about 0.5 to 1.0 disc diameter between the new site of the fovea and the edge of the choriocapillaris defect, thereby rendering recurrent CNVM amenable to thermolaser treatment.
The angle of retinal rotation ranged from 10° to 60°, which was effective in all but one case to translocate the macula outside the area of the RPE defect. Similar angles of rotation are reported by others.9,21,23 The sensory consequences of macular translocation—namely, diplopia and cyclotropia, might be compensated by counter-rotation of the globe.9,23,27 We performed counter-rotation in 27 patients. We noted no statistically significant correction between subjective perception of diplopia and the degree of remaining cyclotropia. Some patients with a remaining cyclotropia of 20° had a positive Bagolini’s striated glasses test and did not complain of diplopia, whereas other patients with cyclotropia of less than 3° and no vertical or horizontal angle did complain of diplopia. In accordance with others9,23,27 our results show that simultaneous perception generally can be achieved through counter-rotation whereas none of our patients achieved simultaneous perception without muscle surgery. Some patients learned to suppress one eye. In our series more patients suppressed the rotated eye than the fellow eye. In some patients the operated eye was suppressed despite better visual acuity. On fixation with the operated eye the patients were then unable to localise objects correctly. Similar findings were reported by other investigators.23,27,28
Similar to Eckardt et al29 we noted no correlation between the risk of diplopia and difference in visual acuity between the eyes in the same individual. We found no correlation between the risk of diplopia and better visual acuity in the rotated eye. These findings do not support the hypothesis that the risk of diplopia can be reduced by selecting patients for macular translocation with low vision in the fellow eye.21 We found no difference in the frequency of diplopia comparing patients with and without compensatory muscle surgery. These findings and the fact that the degree of measured cyclotropia did not correlate with perception of diplopia suggests that muscle surgery is of little help in compensating surgically induced retinal torsion. We speculate that diplopia after macular translocation is in part triggered by distortion of receptive fields and gross changes in muscle mechanics in patients with counter-rotation. This would explain the hampered absolute localisation of images and disturbance of the egocentric localisation. Absolute localisation was not examined here, but should be investigated further with new tests to be developed. While adaptation to altered absolute localisation is possible under monocular viewing conditions, we would expect it to be nearly impossible under binocular viewing conditions.30 Surprisingly, 49% of the patients were able to somehow compensate this problem; 46% of the patients mentioned diplopia, independent from muscle surgery, but only 15% of all patients suffered from disturbing diplopia. Some patients noted diplopia although Bagolini’s striated glasses test was positive or even suppressed. New neurosensory tests are required to help with patient selection for macular translocation. We nevertheless suggest continuation of muscle surgery for macular translocation. Only patients treated by counter-rotation had a chance to achieve simultaneous perception and we did not observe operative complications due to muscle surgery—for example, anterior segment ischaemia.
Despite difficult neurosensory alterations following macular translocation it seems to be possible to stabilise foveal vision over 1 year, which is novel and unique at least for the occult forms of exudative ARMD. We suggest testing our preliminary data by comparing macular translocation for occult CNVM with observation in a randomised trial.
REFERENCES
- 1.Kahn HA, Moorhead HB. Statistics on blindness in the model reporting area 1969–1970. USDHEW publication no (NIH) 1973:73–427.
- 2.Scheider A, Gundisch O, Kampik A. Surgical extraction of subfoveal choroidal new vessels and submacular haemorrhage in age-related macular degeneration: results of a prospective study. Graefes Arch Clin Exp Ophthalmol 1999;237:10–5. [DOI] [PubMed] [Google Scholar]
- 3.Grossniklaus HE, Hutchinson AK, Capone A Jr, et al. Clinicopathologic features of surgically excised choroidal neovascular membranes. Ophthalmology 1994;101:1099–111. [DOI] [PubMed] [Google Scholar]
- 4.Grossniklaus HE, Gass JD. Clinicopathologic correlations of surgically excised type 1 and type 2 submacular choroidal neovascular membranes. Am J Ophthalmol 1998;126:59–69. [DOI] [PubMed] [Google Scholar]
- 5.Lindsey P, Finkelstein D, D’Anna S. Experimental retinal relocation. (ARVO Abstracts) Invest Ophthalmol Vis Sci 1983;24(suppl 3):242. [Google Scholar]
- 6.Tiedeman J, de Juan E Jr, Machemer R et al. Surgical relocation of the macula. (ARVO Abstracts) Invest Ophthalmol Vis Sci 1985;26(suppl):59. [Google Scholar]
- 7.Machemer, R. Steinhorst UH, Retinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 1993;231:635–41. [DOI] [PubMed] [Google Scholar]
- 8.De Juan E Jr, Loewenstein A, Bressler NM, et al. Translocation of the retina for management of subfoveal choroidal neovascularization. II: A preliminary report in humans. Am J Ophthalmol 1998;125:635–46. [DOI] [PubMed] [Google Scholar]
- 9.Eckardt C, Eckardt U, Conrad HG. Macular rotation with and without counter-rotation of the globe in patients with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 1999;237:313–25. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlin JA, Bressler NM, Bressler SB, et al. The use of fundus photographs and fluorescein angiograms in the identification and treatment of choroidal neovascularization in the Macular Photocoagulation Study. The Macular Photocoagulation Study Group. Ophthalmology 1989;96:1526–34. [DOI] [PubMed] [Google Scholar]
- 11.Avery RL, Fekrat S, Hawkins BS, et al. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina 1996;16:183–9. [DOI] [PubMed] [Google Scholar]
- 12.Bennett SR, Folk JC, Blodi CF, et al. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol 1990;109:33–7. [DOI] [PubMed] [Google Scholar]
- 13.Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol 1991;109:1242–57. [PubMed] [Google Scholar]
- 14.Ferris FL, Kassoff A, Bresnick GH, et al. New visual acuity charts for clinical research. Am J Ophthalmol 1982;94:91–6. [PubMed] [Google Scholar]
- 15.Au Eong KG, Pieramici DJ, Fujii GY, et al. Macular translocation: unifying concepts, terminology, and classification. Am J Ophthalmol 2001;131:244–53. [DOI] [PubMed] [Google Scholar]
- 16.Macular Photocoagulation Study Group. Persistent and recurrent neovascularization after laser photocoagulation for subfoveal choroidal neovascularization of age-related macular degeneration. Arch Ophthalmol 1994;112:489–99. [DOI] [PubMed] [Google Scholar]
- 17.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Arch Ophthalmol 1993;111:1200–9. [DOI] [PubMed] [Google Scholar]
- 18.Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Arch Ophthalmol 1999;117:1329–45. [PubMed] [Google Scholar]
- 19.Lambert HM, Capone A, Jr.Aaberg TM, et al. Surgical excision of subfoveal neovascular membranes in age-related macular degeneration. Am J Ophthalmol 1992;113:257–62. [DOI] [PubMed] [Google Scholar]
- 20.Lappas A, Weinberger, A.Foerster, A. et al. Iris pigment epithelial cell translocation in exudative age-related macular degeneration. A pilot study in patients. Graefes Arch Clin Exp Ophthalmol 2000;238:631–41. [DOI] [PubMed] [Google Scholar]
- 21.Seaber JH, Machemer R, Adaptation to monocular torsion after macular translocation. Graefes Arch Clin Exp Ophthalmol 1997;235:76–81. [DOI] [PubMed] [Google Scholar]
- 22.Wolf S, Lappas A, Weinberger AW, et al., Macular translocation for surgical management of subfoveal choroidal neovascularizations in patients with AMD: first results. Graefes Arch Clin Exp Ophthalmol 1999;237:51–7. [DOI] [PubMed] [Google Scholar]
- 23.Fricke J, Neugebauer A, Nobis H, et al. Counterrotation of the globe in macular translocation. Graefes Arch Clin Exp Ophthalmol 2000;238:664–8. [DOI] [PubMed] [Google Scholar]
- 24.RAD Study. Radiation Therapy for Age-related Macular Degeneration. A prospective, randomized, double-masked trial on radiation therapy for neovascular age-related macular degeneration . Ophthalmology 1999;106:2239–47. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya Y, Lewis JM, Hasegawa T, et al. Retinotomy and foveal translocation for surgical management of subfoveal choroidal neovascular membranes. Am J Ophthalmol 1996;122:613–21. [DOI] [PubMed] [Google Scholar]
- 26.Melberg NS, Thomas MA, Burgess DB. The surgical removal of subfoveal choroidal neovascularization. Ingrowth site as a predictor of visual outcome. Retina 1996; 16:190–5. [DOI] [PubMed] [Google Scholar]
- 27.Eckardt U, Eckardt C, Orthoptic problems after macular rotation with and without muscle surgery. Klin Monatsbl Augenheilkd 1998;212:212–7. [DOI] [PubMed] [Google Scholar]
- 28.Fujikado T, Ohji M, Kusaka S, et al. Visual function after foveal translocation with 360-degree retinotomy and simultaneous torsional muscle surgery in patients with myopic neovascular maculopathy. Am J Ophthalmol 2001;131:101–10. [DOI] [PubMed] [Google Scholar]
- 29.Eckardt U, Eckardt C. Diplopie und Konfusion nach Makulatranslokation: Welche Rolle spielt der postoperative Visus? Der Ophthalmologe 2001;98:(Suppl 1):27–8. [Google Scholar]
- 30.Herzau V, Joos E. Untersuchungen von Bewegungen und Stellungsfehlern der Augen um ihre sagittale Achse. Z Prakt Augnheilkd 1983;4:279. [Google Scholar]