Abstract
Background: The Humphrey field analyser (HFA), Humphrey-Zeiss frequency doubling perimeter, and the Medmont automated perimeter (MAP) are three commonly used automated perimeters with threshold achromatic methodologies. Visual field loss may be detected earlier with strategies that target cell lines with reduced redundancy or which suffer selective damage.
Method: To compare these three perimeters, 63 subjects who were glaucoma suspects, ocular hypertensives, glaucoma patients, or normal controls were recruited selectively. All subjects underwent testing using MAP central threshold, MAP flicker perimetry, HFA full threshold, HFA SITA perimetry, HFA short wavelength perimetry (SWAP), and frequency doubling perimetry (FDP). After visual field testing, equivalent tests were compared: MAP central threshold with HFA full threshold and HFA SITA perimetry; Medmont flicker perimetry with HFA SWAP and FDP.
Results: On analysis of the MAP central threshold a kappa statistic and an area under the receiver operator curve (AUC) of 0.90 and 0.94, respectively, were found compared with HFA full threshold strategies, and 0.87 and 0.92 respectively, compared with HFA SITA. For MAP flicker a kappa statistic and an AUC of 0.65 and 0.81, respectively, were found compared with HFA SWAP and 0.87 and 0.96, respectively, compared with FDP. A quadrant analysis and comparison of mean defect between tests was also highly significant.
Conclusion: Medmont and Humphrey perimeters correlated well; both may be used for clinical and research purposes with similar confidence.
Keywords: Medmont perimetry, Humphrey perimetry, visual fields
A utomated perimetry provides a reliable, accurate, and reproducible method of visual field testing.1–4 One of the most important benefits of automation has been the ability to standardise test procedures.5 Conventional static achromatic automated perimetry (AAP), however, may only show visual field losses if up to 50% of the ganglion cells are lost.6,7 More recently, tests of early visual field loss, such as flicker perimetry, short wavelength automated perimetry (SWAP), and frequency doubling perimetry (FDP) have detected visual field losses before achromatic perimetry.8–12
Three commonly utilised automated perimetric systems in Australia are the Humphrey field analyser (HFA), the Humphrey-Zeiss frequency doubling perimeter, and the Medmont automated perimeter (MAP). The HFA uses a hemispheric projection bowl of radius 33 cm with a uniform background illumination of 31.5 apostilbs (asb). A projection device presents a light stimulus at specified points in the visual field. While stimulus size may be varied, the most common used has a diameter of 0.43° (Goldmann size III); it is usually white, but may be red, blue, or green.1 To test for fixation loss, a stimulus is presented at the blind spot intermittently through the test; this should yield no response if a patient is fixating correctly. In some models, patient fixation can be monitored directly by the technician using a video camera.
The HFA can be used in full threshold mode, which uses the threshold levels determined at one primary point in each quadrant as a starting level for neighbouring points. In turn, these are used as starting intensity levels for their neighbouring points, and so on. At each point, stimulus intensity is increased in 4 dB steps until threshold is crossed from non-seeing to seeing, and then reduced in 2 dB steps to check and refine the accuracy of the assessment.1
Test time can be reduced by the utilisation of a more recently developed program called Swedish Interactive Thresholding Algorithm (SITA).13,14 This testing strategy continuously estimates both the threshold values and the measurement errors of those values using Bayesian posterior probability calculations.13,14 Continuously modified staircase procedures are used to alter stimulus intensities at the test locations; these staircases are interrupted when measurement errors are reduced to predetermined levels. This coupled with a reduction in the number of catch trials for determination of frequencies of false positive answers and an increase in speed of stimulus presentation as permitted by patient responsiveness leads to significant reductions in test time while retaining accuracy.13,14
For short wavelength automated perimetry (SWAP), the HFA background illumination is altered to a yellow light at 100 cd/m2 with the target a blue stimulus (440 nm), Goldmann size V (1.72° diameter). Blue-on cell pathways can be tested in isolation when the yellow background effectively reduces the sensitivities of red and green cones.15 Johnson postulated16 that as blue-on cells are the largest colour sensitive cells,17 they are more likely to be lost selectively in glaucoma. Furthermore, because blue-on cells have larger receptive fields and are fewer in number,17 there is less overlap than there is for red and green receptive fields. This reduced redundancy,16 postulated Johnson, allows these areas of axonal loss to be detected by SWAP before AAP.8,9
MAP utilises a 30 cm radius perimetric bowl with 164 green light emitting diodes (wavelength 565 nm) acting as the stimulus. The background illuminating light source maintains an illumination of 10 asb. Unable to be varied in size or hue, each stimulus is an equivalent Goldmann size III. Fixation is monitored with stimulus points presented within the blind spot. Central fixation is not directly video monitored.18
Central threshold testing is performed in a pattern, which tests the visual field to 22° from central fixation superiorly, inferiorly, and temporally, and to 30° nasally. It uses a testing strategy which decreases stimulus intensity by 6 dB until threshold is crossed and then reverses in steps of 3 dB until the threshold sensitivity at a particular test location is confirmed.18,19
The MAP may be modified to perform flicker perimetry. Targets are presented in a 22° radius of the central visual field at frequencies varying from 9–18 Hz (depending on target eccentricity). Target luminance is varied in order to determine threshold.20 False positive responses were tested with static stimuli. Flicker perception is thought to be conveyed through the magnocellular (M) pathway.16 Being larger in size,21–23 M cells may be lost earlier in glaucoma by means of selective loss16 and, as they only constitute 25% of ganglion cells,21–23 tests which target them, such as flicker perimetry, may be more likely to detect these losses earlier then conventional perimetry because of reduced redundancy.16
Frequency doubling perimetry (FDP) uses 10° square target zones, consisting of alternating vertical white and black stripes (spatial frequency 0.25 cycles per degree, cpd). These stripes are then reversed at a rate of 25 Hz and the contrast between the stripes is changed to determine sensitivity. The test has been used to detect established visual field losses in the same way that HFA AAP does.24 However, recently its ability to detect early visual field losses has been explored.10–12
FDP seems to target a subset of the magnocellular pathway with non-linear or Y-like properties (My pathway).16,25 This pathway makes up only approximately 15% of the magnocellular system.26–28 As larger cells and relatively few in number, they may be more susceptible to selective loss and reduced redundancy, allowing an earlier detection of any fall in cell numbers.10–12,16
METHOD
A total of 63 patients were recruited selectively from a patient population attending an urban glaucoma clinic. None of these had diabetes, cataract, or corneal or retinal disease, which could affect test results. Patients were glaucoma suspects, had ocular hypertension, open angle glaucoma, or were control subjects. The glaucoma suspect group had a family history of glaucoma or had suspicious discs but with no definite structural changes and normal intraocular pressure (IOP <21 mm Hg) and visual fields. Ocular hypertension was diagnosed as IOP ≥21 mm Hg on at least three occasions with no previous field changes on full threshold HFA testing and no evidence of glaucomatous optic neuropathy. Open angle glaucoma patients had glaucomatous optic disc changes with or without characteristic visual field abnormality on 24-2 HFA full threshold testing. Among patients with glaucoma, ocular hypertension, or glaucoma suspects, only those patients were included who had at least two consecutive visual fields, performed within the past 2 years. One eye from each patient was considered. When both eyes were eligible a random choice was made.
Medmont, HFA, and FDP were performed in random order after informed consent was obtained. The Humphrey field analyser II (Carl Zeiss, Dublin, CA, USA) was used to perform central 24-2 full threshold, central 24-2 SITA standard and central 24-2 SWAP tests. The determination of a significant scotoma was based on the pattern deviation of the HFA probability plot. Visual field loss was considered significant if it had a pattern typical of glaucoma and occurred in a field with five or more points of p <5%, with a cluster of three or more abnormal points of p <5%, or two or more points of p <1%.
The Medmont M600 automated perimeter (Medmont, Camberwell, Victoria, Australia) was used to perform central 30 degree threshold and 15/22 flicker perimetry. The determination of a significant scotoma was based on the age normal plot of the Medmont M600 printout. Before the analysis, both strict and loose scotoma criteria were developed. For the former, visual field loss was considered significant if it had a pattern typical of glaucoma and occurred in a field with six or more points of >6 dB depressed, with a cluster of four or more abnormal points of >6 dB depressed, or three or more points of >18 dB depressed. For the latter, a scotoma was considered significant if it had a pattern typical of glaucoma and occurred in a cluster of three or more points depressed >6 dB, or two or more points >18 dB.
The Humphrey-Zeiss frequency doubling perimeter (Carl Zeiss, Dublin, CA, USA) was used to perform N-30 full threshold testing. Visual field loss was considered significant if there were two or more adjacent zones of p <5%, or one zone of p <1% on the FDP pattern deviation. In all cases, fields were considered reliable if there were less than 33% false negative and false positive errors and less than 20% fixation losses.
All patients underwent all five perimetric tests. In order to compare Medmont with other equivalent perimetric methods we compared Medmont central 30 degree threshold perimetry with HFA central 24-2 full threshold and central 24-2 SITA standard. Medmont 15/22 flicker perimetry was compared with HFA central 24-2 SWAP and FDP N-30 full threshold.
The comparisons were multifactorial. To demonstrate the ability of Medmont to localise scotomas in the same areas as the Humphrey perimeter, a quadrant analysis was performed. The abnormal points within each quadrant were totalled and tests were compared with simple linear regression. To illustrate any significance in Medmont’s global indices, mean deviations were compared between tests using a simple linear regression. A kappa statistic was calculated to demonstrate the degree of agreement the tests. Finally, an area under the receiver operator curve (ROC) curve was calculated for each pair of tests being compared.
Statistical Analysis System (SAS Institute Inc, Cary, NC, USA) was used for statistical analysis including frequency tables, descriptive statistics, Student’s t test and simple linear regression. Excel 97 (Microsoft, Redmond, WA, USA) was used in the calculation of area under the ROC curve and the kappa statistic.
RESULTS
Consisting of 34 females (54%) and 29 males (46%), the average age of the participants was 60 years (standard deviation 13 years). There were 15 controls (24%), eight glaucoma suspects (13%), eight ocular hypertensives (13%), and 32 open angle glaucoma patients (51%). A description of these groups and the amount of visual field loss is shown in Tables 1 and 2 respectively. The mean test times for Humphrey and Medmont perimeters are displayed in Table 3.
Table 1.
Description of patients within the study groups
| Group | Number | Males (%) | Mean age (SD) | Range |
| Control | 15 | 7 (47%) | 52 years (15 years) | 29–75 years |
| Glaucoma suspects | 8 | 5 (63%) | 56 years (16 years) | 35–77 years |
| Ocular hypertension | 8 | 1 (13%) | 60 years (9 years) | 47–74 years |
| Open angle glaucoma | 32 | 16 (50%) | 64 years (9 years) | 41–79 years |
Table 2.
Description the amount of visual field loss for patients within the study groups
| Humphrey full threshold MD | Range | ||
| Group | Average | (SD) | |
| Control | −0.75 | (1.05) | 0.59 to −2.34 |
| Glaucoma suspects | −0.66 | (1.23) | 1.57 to −1.72 |
| Ocular hypertension | −1.19 | (2.39) | 0.90 to −6.41 |
| Open angle glaucoma | −8.20 | (7.51) | 1.04 to −26.58 |
Table 3.
Mean test time (SD) for Humphrey and Medmont perimetry
| Mean test time | SD | |
| Medmont central threshold | 10 minutes 51 seconds | 51 seconds |
| Medmont flicker perimetry | 9 minutes 47 seconds | 1 minutes 6 seconds |
| Humphrey full threshold | 10 minutes 43 seconds | 1 minutes 26 seconds |
| Humphrey SITA | 5 minutes 44 seconds | 1 minutes 12 seconds |
| Short wavelength automated perimetry | 10 minutes 35 seconds | 1 minutes 43 seconds |
| Frequency doubling perimetry | 5 minutes 8 seconds | 30 seconds |
When Medmont central threshold was compared with HFA full threshold (Tables 4 and 5), a scotoma analysis yielded a kappa statistic of 0.90 under the strict criteria and 0.72 under the loose criteria. The area under the ROC curve was 0.94, indicating a strong correlation between the two tests (Table 5). When Medmont central threshold was compared with HFA SITA standard (Tables 4 and 5), this showed a kappa statistic of 0.87 under the strict criteria and 0.72 under the loose criteria. The area under the ROC curve was 0.92 (Table 5), again indicating a strong correlation. These findings were supported by the quadrant analysis and mean deviation r2 statistics, which also show highly significant correlations between Medmont central threshold and HFA full threshold or SITA standard. HFA SITA was significantly faster than Medmont central threshold (p<0.001), but Medmont central threshold and HFA full threshold had no significant difference in test time (p=0.53).
Table 4.
Numbers of normal and abnormal Medmont central threshold, Humphrey full threshold, and SITA in the patient sample
| Humphrey full threshold | Humphrey SITA | |||
| Abnormal | Normal | Abnormal | Normal | |
| Medmont central threshold (strict) | ||||
| Abnormal | 24 | 2 | 25 | 2 |
| Normal | 1 | 36 | 2 | 34 |
| Medmont central threshold (loose) | ||||
| Abnormal | 25 | 9 | 26 | 8 |
| Normal | 0 | 29 | 1 | 28 |
Table 5.
Comparison of Medmont central threshold with Humphrey full threshold and Humphrey SITA showing, kappa statistic, area under the ROC curve (AUC), quadrant analysis, and mean defect correlation
| Medmont central threshold (strict) | Medmont central threshold (loose) | |||
| Compared with | Compared with | |||
| Humphrey full threshold | Humphreys SITA | Humphrey full threshold | Humphrey SITA | |
| Kappa statistic | 0.90 | 0.87 | 0.72 | 0.72 |
| AUC | 0.94 | 0.92 | ||
| Sector correlation (r2 statistic): | ||||
| Superonasal | 0.86** | 0.85** | ||
| Superotemporal | 0.80** | 0.72** | ||
| Inferonasal | 0.69** | 0.62** | ||
| Inferotemporal | 0.29** | 0.23** | ||
| Mean defect correlation (r2 statistic): | 0.89** | 0.88** | ||
*p<0.001, **p<0.0001.
After Medmont flicker was compared with HFA SWAP (Table 6), this yielded a kappa statistic of 0.65 and 0.62 for the strict and loose criteria respectively. The area under the ROC curve was 0.81 (Table 7). When compared with FDP (Table 6) the kappa statistic was 0.87 and 0.78 for the strict and loose criteria respectively. The area under the ROC curve was 0.96 (Table 7). The r2 statistics from the quadrant and mean deviation analysis were also highly significant, although more so for FDP than for SWAP. FDP was significantly faster than Medmont flicker (p<0.001), but Medmont flicker was significantly faster than SWAP (p<0.01).
Table 6.
Numbers of normal and abnormal Medmont flicker perimetry, Humphrey short wavelength perimetry, and Humphrey frequency doubling perimetry in the patient sample
| Humphrey short wavelength perimetry | Humphrey frequency doubling perimetry | |||
| Abnormal | Normal | Abnormal | Normal | |
| Medmont flicker (strict) | ||||
| Abnormal | 23 | 8 | 29 | 2 |
| Normal | 3 | 29 | 2 | 30 |
| Medmont flicker (loose) | ||||
| Abnormal | 27 | 10 | 29 | 5 |
| Normal | 2 | 24 | 2 | 27 |
Table 7.
Comparison of Medmont flicker perimetry with Humphrey short wavelength perimetry and Humphrey frequency doubling perimetry showing kappa statistic, area under the ROC curve (AUC), quadrant analysis, and mean defect correlation
| Medmont flicker (strict) | Medmont flicker (loose) | |||
| Compared with | Compared with | |||
| Humphrey short wavelength perimetry | Humphrey frequency doubling perimetry | Humphrey SWAP | Humphrey FDP | |
| Kappa statistic | 0.65 | 0.87 | 0.62 | 0.78 |
| AUC | 0.81 | 0.96 | ||
| Sector correlation (r2 statistic): | ||||
| Superonasal | 0.48** | 0.67** | ||
| Superotemporal | 0.25** | 0.79** | ||
| Inferonasal | 0.17* | 0.64** | ||
| Inferotemporal | 0.02 | 0.72** | ||
| Mean defect correlation (r2 statistic): | 0.57** | 0.79** | ||
*p<0.001, **p<0.0001.
DISCUSSION
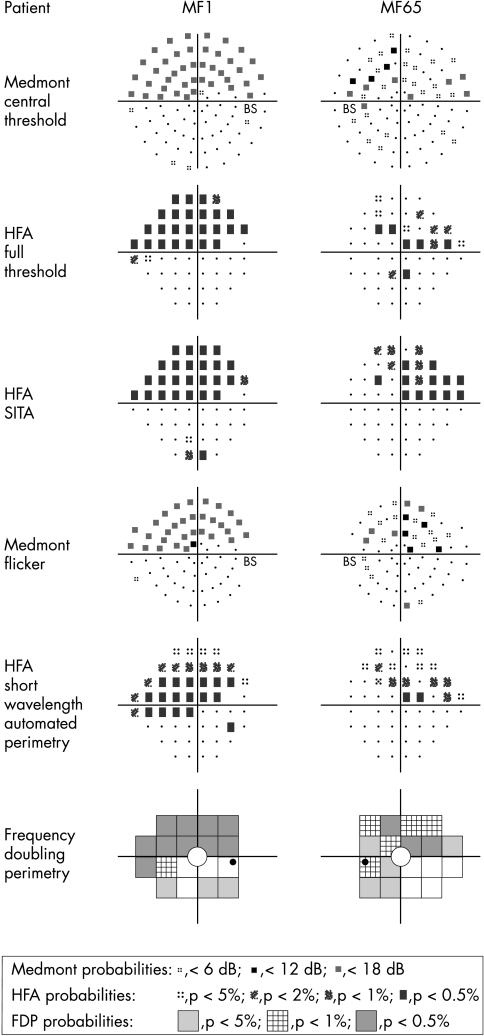
The Medmont M600 perimeter, brought out in the late 1980s,18 was promoted as quicker than HFA in performing a full threshold analysis of the visual field.18 Possibly, recent advances in HFA full threshold analysis have meant that it can be performed in the same time as Medmont. SITA dramatically reduces test time. In this study, Medmont yielded results comparable with those of the HFA and the FDP (Fig 1).
Figure 1.
Pattern deviation from two patients (MF1, MF65).
Although Medmont’s step size is larger than HFA (6 dB versus 4 dB) this has been reported not to cause any difference in accuracy on measuring scotoma size.18,19 Other Medmont parameters are similar to HFA18 and this is reflected in the high correlation between the two kinds of achromatic perimetry.
A flickering stimulus will target the magnocellular pathway and this has been the basis for many tests of visual field loss,16,29,30 the most recent being FDP. Medmont flicker correlates strongly with FDP in all of the parameters tested. As Medmont flicker targets a subgroup of ganglion cells which are felt to be lost early in glaucoma,16 it may be detecting visual field losses that may precede detection with achromatic perimetry. Support for this is seen not only in the correlation with FDP but also with SWAP which has the ability to detect scotomas before they become manifest on AAP.8,9 While Medmont flicker cannot be performed as rapidly as FDP, it may be quicker than SWAP.
We conclude that Medmont and Humphrey perimetry correlated favourably with one another, and therefore, both may be used for clinical and research purposes with similar confidence.
Supplementary Material
Acknowledgments
We would like to thank the staff at Eye Associates for their support and assistance.
Abbreviations
AAP, achromatic automated perimetry
AUC, area under the receiver operator curve
FDP, frequency doubling perimetry
HFA, Humphrey field analyser
MAP, Medmont automated perimeter
SITA, Swedish Interactive Thresholding Algorithm
SWAP, short wavelength automated perimetry
REFERENCES
- 1.Beck RW, Berstrom TJ, Lichter PR. A clinical comparison of visual field testing with a new automated perimeter, the Humphrey field analyzer, and the Goldmann perimeter. Ophthalmology 1985;92:77–82. [DOI] [PubMed] [Google Scholar]
- 2.Katz J, Quigley HA, Sommer A. Repeatability of the glaucoma hemifield test in automated perimetry. Invest Ophthalmol Vis Sci 1995;36:1658–64. [PubMed] [Google Scholar]
- 3.McCrary JA, Feigon J. Computerized perimetry in neuro-ophthalmology. Ophthalmology 1979;86:1287–301. [DOI] [PubMed] [Google Scholar]
- 4.Johnson CA, Keltner JL, Balestrery FG. Suprathreshold static perimetry in glaucoma and other optic nerve disease. Ophthalmology 1979;86:1278–86. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CA. Role of automation in new instrumentation. Optom Vis Sci 1993;70:288–98. [DOI] [PubMed] [Google Scholar]
- 6.Quigley HA, Addicks EM, Green R. Optic nerve damage in human glaucoma. Arch Ophthalmol 1982;100:135–46. [DOI] [PubMed] [Google Scholar]
- 7.Harwerth RS, Carter-Dawson L, Shen F, et al. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci 1999;40:2242–50. [PubMed] [Google Scholar]
- 8.Demirel S, Johnson CA. Short wavelength automated perimetry (SWAP) in ophthalmic practice. J Am Opt Assoc 1996;67:451–6. [PubMed] [Google Scholar]
- 9.Johnson CA, Adams AJ, Casson EJ, et al. Blue-on-yellow perimetry can predict the development of glaucomatous visual field loss. Arch Ophthalmol 1993;111:645–50. [DOI] [PubMed] [Google Scholar]
- 10.Johnson CA, Cioffi GA, Van Buskirk EM. Frequency doubling technology perimetry using a 24-2 stimulus presentation pattern. Optom Vis Sci 1999;76:571. [DOI] [PubMed] [Google Scholar]
- 11.Brusini P, Busatto P. Frequency doubling perimetry in glaucoma early diagnosis. Acta Ophthalmol (Scand) 1998;227:23–4. [DOI] [PubMed] [Google Scholar]
- 12.Landers J, Goldberg I, Graham S. A comparison of short wavelength automated perimetry with frequency doubling perimetry for the early detection of visual field loss in ocular hypertension. Clin Exp Ophthalmol 2000;28:248–52. [DOI] [PubMed] [Google Scholar]
- 13.Sharma AK, Goldberg I, Graham SL, et al. Comparison of the Humphrey Swedish Interactive Thresholding Algorithm (SITA) and full threshold strategies. J Glaucoma 2000;9:20–7. [DOI] [PubMed] [Google Scholar]
- 14.Bengtsson B, Olsson J, Heijl A, et al. A new generation of algorithms for computerised threshold perimetry, SITA. Acta Ophthalmol (Scand) 1997;75:368–75. [DOI] [PubMed] [Google Scholar]
- 15.Sample PA, Johnson CA, Haegerstrom-Portnoy G, et al. Optimum parameters for short-wavelength automated perimetry. J Glaucoma 1996;5:375–83. [PubMed] [Google Scholar]
- 16.Johnson CA. Early losses of visual function in glaucoma. Optom Vis Sci 1995;72:359–70. [DOI] [PubMed] [Google Scholar]
- 17.De Monasterio FM. Asymmetry of on- and off-pathways of blue-sensitive cones of the retina of macaques. Brain Res 1979;166:39–48. [DOI] [PubMed] [Google Scholar]
- 18.Vingrys AJ, Helfrich KA. The Opticom M600: a new LED automated perimeter. Clin Exp Opt 1990;73:3–17. [Google Scholar]
- 19.Pye D, Herse P, Nguyen H, et al. Conversion factor for comparison of data from Humphrey and Medmont automated perimeters. Clin Exp Opt 1999;82:11–13. [DOI] [PubMed] [Google Scholar]
- 20.McKendrick AM, Vingrys AJ, Badcock DR, et al. Visual field losses in subjects with migraine headaches. Invest Ophthalmol Vis Sci 2000;41:1239–47. [PubMed] [Google Scholar]
- 21.Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci 1991;32:484–91. [PubMed] [Google Scholar]
- 22.Chaturvedi N, Hedley-Whyte ET, Dreyer EB. Lateral geniculate nucleus in glaucoma. Am J Ophthalmol 1993;116:182–8. [DOI] [PubMed] [Google Scholar]
- 23.Maddess T, Hemmi JM, James AC. Evidence for spatial aliasing effects in the y-like cells of the magnocellular visual pathway. Vis Res 1992;38:1843–59. [DOI] [PubMed] [Google Scholar]
- 24.Burnstein Y, Ellish N, Magbalov M, et al. Comparison of frequency doubling perimetry with Humphrey visual field analysis in a glaucoma practice. Am J Ophthalmol 2000;129:328–33. [DOI] [PubMed] [Google Scholar]
- 25.Sponsel WE, Argango S, Trigo Y, et al. Clinical classification of glaucomatous visual field loss by frequency doubling perimetry. Am J Ophthalmol 1998;125:830–6. [DOI] [PubMed] [Google Scholar]
- 26.Maddess T, Hemmi JM, James AC. Evidence for spatial aliasing effects in the y-like cells of the magnocellular visual pathway. Vis Res 1992;38:1843–59. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan E, Shapley RM. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol 1982;330:125–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maddess T, Henry GH. Performance of nonlinear visual units in ocular hypertension and glaucoma. Clin Vis Sci 1992;7:371–83. [Google Scholar]
- 29.Graham SL, Drance SM, Chauhan BC, et al. Comparison of psychophysical and electrophysiological testing in early glaucoma. Invest Ophthalmol Vis Sci 1996;37:2651–62. [PubMed] [Google Scholar]
- 30.Casson EJ, Johnson CA, Shapiro LR. Longitudinal comparison of temporal-modulation perimetry with white-on-white and blue-on-yellow perimetry in ocular hypertension and early glaucoma. J Opt Soc Am 1993;10:1792–806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.