Abstract
Aim: To assess the safety and predictability of photorefractive keratotomy (PRK) and laser in situ keratomileusis (LASIK) based on preoperative corneal topography.
Methods: A non-randomised comparative study was carried out on 84 eyes that presented with topographic abnormalities before undergoing PRK (n = 44) or LASIK (n = 40) procedures. 84 spherical equivalent paired normal eyes served as the control group. Either PRK or LASIK procedures were performed on 168 eyes using the Summit apex plus excimer laser. Topographic abnormalities, including apex displacement (AD), increased asphericity (AS), meridional irregularity (MI), increased inferior-superior asymmetry (IS), increased curvature (CU), and combined features (CO), were assessed preoperatively using the EyeSys analysis system. Safety and predictability of the two procedures were defined as a postoperative visual acuity of 20/40 or better and the loss of one or more lines of spectacle corrected visual acuity (SCVA).
Results: All patients were followed for 6 months. There was a significant loss of best corrected visual acuity in the PRK-AD (p<0.001), PRK-CO (p<0.05), and LASIK-AS (p<0.001) patients. The number of eyes within plus or minus 1.0D of the surgical plan postoperatively was similar in all groups.
Conclusion: These data suggest that although predictability was similar, PRK and LASIK performed in corneas with topographic abnormalities might cause loss of vision.
Keywords: photorefractive keratotomy, LASIK, corneal topography
Refractive surgery is an increasingly popular procedure to decrease spectacle or contact lens dependency. The risks of refractive surgery are low on an individual basis, but the impact on the population must be carefully evaluated by the medical community.1
Photorefractive keratectomy (PRK) and laser in situ keratomileusis (LASIK) are two refractive procedures currently leading the field. The number of LASIK procedures has increased and far surpasses the number of PRK procedures owing to faster visual recovery, less pain, and greater ametropic range capability. The intraoperative risks related to LASIK are intrinsically greater than those related to PRK.1 Postoperative complications related to PRK include haze and regression which have become major limitations of the procedure. Long term complications related to LASIK include ectasia due to corneal weakening, which is not fully understood or well controlled.2
The prevention of complications is a major goal in these elective procedures. Realistic patient expectations, night vision disabilities, and transient discomfort must be discussed with all patients before surgery, and a comprehensive ophthalmological examination should be performed. Current technology allows us to diagnose a limited range of corneal diseases, therefore the potential visual results of the procedures in abnormal eyes are not clear.
Approximately 5% of all candidates for refractive surgery present with clinical keratoconus, diagnosed by either slit lamp examination or the corneal reflex (placido disc or keratometry).3,4 The literature presents controversial results in two small case series of keratoconus patients submitted to PRK without adequate predictability or safety.5,6 For this reason LASIK and PRK are contraindicated in keratoconus.
Many indexes have been suggested to diagnose possible keratoconic corneas. The first index was proposed by Rabinowitz and is known as the I/S index, in which differences in the 3 mm superior/inferior area of greater than 1.3D carries a greater risk of keratoconus.7 Rabinowitz recently presented the KISA index to predict keratoconus, which combines several features including central keratometry, steepening, and skewness of the flattest semi-meridians.8 Smolek et al also combined several variables as sectorial curvatures to design software that has been incorporated in some topography machines and to assess the risk of keratoconus.9
Even with significant progress in this field, some cases do not fit all the criteria, leaving the surgeon in doubt about the keratoconus diagnosis or other topographic abnormalities. The objective of this study was to assess the visual outcome of excimer laser refractive surgery in these eyes.
METHODS
We performed a comparative interventional study of patients who presented with abnormal corneal topographies before PRK or LASIK procedures performed between April 1996 and August 1999. All patients were examined at the refractive surgery clinic of the department of ophthalmology of the Federal University of São Paulo, UNIFESP, Brazil.
Patients were examined preoperatively to obtain full medical history, measurement of visual acuity with spectacle corrected visual acuity (SCVA) and without spectacle correction, cycloplegic refraction, slit lamp biomicroscopy, applanation tonometry, fundus examination, corneal topography, and ultrasonic pachymetry.
Exclusion criteria for surgery included any systemic or ocular disease, such as clinically manifested keratoconus (with any slit lamp signs or more than two of the above listed topographic criteria), cataract, glaucoma, or retinal disorders. Patients with peripheral retinal degeneration were evaluated by specialists and subjected to argon photocoagulation before the refractive procedure when indicated. Informed consent was obtained from all patients following a full explanation of the known risks related to the procedure and its complications.
The procedures were performed according to the cycloplegic refraction, using the Summit Apex Plus excimer laser (Summit Technology, Waltham, MA, USA) and the lamellar keratotomy was performed with the automated corneal shaper (Chiron Vision, Emmerville, USA) and LSK evolution 2 (Moria, Antony, France), performed by qualified doctors from UNIFESP after extensive discussion of each clinical case.
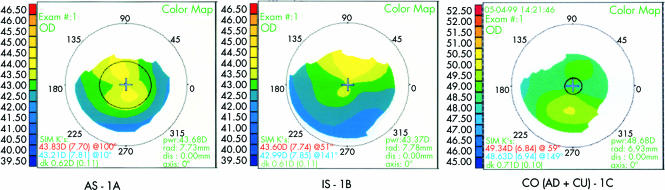
Inclusion criteria for analysis were apex displacement up to 1.5D (AD), asphericity of more than 0.25D/mm (AS), meridional irregularity of more than 15 degrees (MI), inferior-superior asymmetry of at least 1.5D (IS), and corneal curvature of at least 47D (CU) (Fig 1).
Figure 1.
Examples of abnormal corneal topographies grouped as AS - 1A, 1S - 1B, and a combination of AD and CU - 1C.
All the patients were followed for 6 months. Eighty four eyes of patients with no preoperative corneal topography irregularities who elected to have the same surgeries (n = 44 PRK, 40 LASIK) comprised the control group.
A paired t test and the χ2 test were used for statistical analysis. A p value of less than 0.05 was considered to be statistically significant.
RESULTS
Forty four PRK and 40 LASIK patients were selected from 6000 patients with various topographies according to the inclusion criteria and compared with an equal number of patients in the control groups. Both groups were comparable statistically (p<0.05) regarding sex, SCVA, and average central stimulated keratometry.
Only one eye from each patient was analysed, usually the more irregular. Other than the preoperative cylinder component in the PRK group and postoperative spherical equivalent in the LASIK group, the refractive data were similar among the control and study groups (Table 1). Two PRK and four LASIK patients presented with a combination of two topographic irregularities (Table 2).
Table 1.
Spherical equivalent (SE) and cylindrical component (CC) among PRK and LASIK patients in the preoperative and postoperative periods
| Preoperative spherical equivalent | Preoperative cylindrical component | Postoperative spherical equivalent | Postoperative cylindrical component | |
| Average (SD) | Average (SD) | Average (SD) | Average (SD) | |
| PRK controls | −3.08 (1.04) | −1.02 (0.60) | 0.41 (0.66) | −0.61 (0.27) |
| PRK, irregular topography | −3.00 (1.19) | −0.88 (0.74) | 0.30 (0.72) | −0.68 (0.40) |
| LASIK controls | −6.64 (2.57) | −1.47 (1.05)* | −0.13 (1.04)** | −0.75 (0.61) |
| LASIK, irregular topography | −6.03 (2.67) | −2.06 (−1.41)* | 0.04 (1.01)** | −0.9 (0.59) |
*Statistical difference p<0.05.
Table 2.
Topographic pattern distribution regarding the surgical procedure
| Apex displacement (AD) | Asphericity (AS) | Meridional irregularity (MI) | Asymmetry (IS) | Curvature (CU) | Combined (CO) | Total | |
| PRK | 24 | 4 | 4 | 5 | 5 | 2 | 44 |
| LASIK | 9 | 13 | 3 | 3 | 8 | 4 | 40 |
Predictability, defined as the number of eyes within plus or minus 1D of the surgical plan, was not different among groups or subgroups.
Efficacy was defined as an achieved uncorrected visual acuity of 20/40 or better using the Snellen chart. In the PRK group, efficacy in eyes with irregular topography and their matched controls did not differ. In the LASIK group, however, the efficacy differed significantly between eyes with topographic irregularities and controls (p<0.05). The incidence of increased asphericity was the only criterion that was significantly different among subgroups of eyes with irregular topography receiving LASIK (p = 0.0018).
Safety was assessed by the loss of lines of SCVA at the last follow up visit. There was a statistically significant vision loss among groups and subgroups (Table 3). If two or more lines were used as the criterion, there were no differences among groups (p = 0.06).
Table 3.
Number of eyes that lost 1 or more lines of SCVA in the subgroups of irregular topography patients and the control group
| AD | AS | MI | IS | CU | CO | Total | |
| PRK, irregular topography | 6* | 0 | 1 | 0 | 1 | 1* | 9 |
| PRK controls | – | – | – | – | – | – | 0 |
| LASIK, irregular topography | 4 | 9* | 1 | 2 | 3 | 2 | 21 |
| LASIK controls | – | – | – | – | – | – | 6 |
| Combined PRK + LASIK patients, irregular topography | 10 | 9 | 2 | 2 | 4 | 3 | 30* |
| Combined PRK + LASIK patients, regular topography | – | – | – | – | – | – | 6 |
*Statistical difference p<0.05.
DISCUSSION
Several studies have demonstrated the safety of refractive procedures. Based on the number of eyes that have lost lines of vision, public agencies such as the Food and Drug Administration have approved refractive procedures for clinical use. Safety is the most important issue compared to efficacy and predictability, to evaluate whether a refractive procedure should be considered for the patient.10
A procedure that results in a loss of less than 5% or one to two lines of vision, as in the control group of the present study, is considered to be safe. None of the control eyes in the PRK group lost SCVA. In contrast, 35% of the patients who presented with irregular topographies before either PRK or LASIK procedures lost at least one line of SCVA.
There was a statistically significant loss of one line. Thus, a significant number of eyes did not recover their SCVA after the procedure if there were previous irregular corneal topographies. These data must be available to allow the patient, the doctor, and the ophthalmic community to decide whether such corneas should undergo refractive surgery. The literature indicates a 0–24% loss of one or more lines in patients with topographical abnormalities; however, none of these studies compared the results with a control group.11–14
Most convergence power of the ocular system arises from the anterior corneal surface, therefore small modifications in its shape produce substantial refractive changes. This is the main reason why the cornea is chosen for ocular refractive remodelling. Optical methods, such as the placido disc evaluation invented in the 19th century, are still used to evaluate and diagnose refractive changes of the cornea. Attempts to use computerised slits of light, such as by the Orbscan I (Orbtek, Salt Lake City, UT, USA) have failed and therefore the current version of this machine, the Orbscan II (Bausch and Lomb, Rochester, NY, USA), incorporates the corneal reflex from placido disc devices to increase measurement reproducibility.
Corneal topography limitations are well known and derive from the fact that there are no gold standards to model the actual corneal shape. Furthermore, tear film and epithelium are also sources of variability. Inaccurate measurement distances, mathematical interpolations, and lack of central reading are also factors. Even so corneal topography is still the best method to achieve either qualitative or quantitative data from the anterior surface of the cornea.
Most refractive surgeons rely on corneal topography to determine whether to perform surgery on highly irregular and asymmetrical astigmatisms. Such corneas behaved unexpectedly when submitted to radial keratotomy in the past and currently there are not enough data to draw conclusions regarding the safety, efficacy, predictability, and stability of surgery in these patients.3
Classic keratoconus due to anatomical thinning of the cornea might accelerate biomechanical instability and produce clinical ectasia. More subtle features, such as corneal multifocality, might prevent 20/20 vision in the patient even before surgery and might further decrease SCVA after the procedure.15
We assumed that no patients in the study had a classic diagnosis of keratoconus, but it is indeed possible that the present technology fails to detect this disease. The only possibility of having this dilemma solved would be to follow the patients and observe for abnormal ectasia rates.3,9 Genetic probes might solve this dilemma in the future.16
Assuming no biomechanical instability in the short term, the focal point of our findings is the physiological optics of these corneas. Preoperatively, visual acuity was comparable in control patients undergoing either PRK or LASIK. This might be due to the fact that our brain “sharpens” images that are not too blurred.17 Contrast sensitivity may also refine these findings.
Postoperatively, there was a significant decrease in visual acuity (efficacy) in the eyes with irregular topography that underwent the LASIK procedure. Applegate recently demonstrated that the cornea is responsible for the large number of aberrations in the optic pathway, and this might explain the overall decrease in visual function under high contrast conditions.17 A cornea with increased asphericity generates lower contrast sensitivity than one with increased regular curvature, because of potential multifocal properties and additional spherical aberrations. Epithelium remodelling may be an explanation for the better efficacy of PRK than LASIK.
Holladay discussed the importance of changing the prolate shape of the cornea to an oblate shape after corneal refractive surgery, and related this change to a decrease in contrast sensitivity.18 Our results indicated a loss of lines in the whole PRK, LASIK, or combined groups with isolated apex displacement, increased asphericity, and multiple factors. The small number of isolated cases limits the conclusion that individual factors might lead to a loss of lines but, nevertheless, eyes with abnormal topographic corneas lost more lines of vision than the control eyes.
In conclusion, corneal photorefractive procedures should be avoided in cases of corneal irregularity until further evidence assures adequate safety for such patients.
Acknowledgments
This paper was presented in part at the 2001 American Academy of Ophthalmology meeting, New Orleans, LO, USA.
Partially supported by the IPEPO.
REFERENCES
- 1.Johnson JD, Azar DT. Surgically induced topographical abnormalities after LASIK: management of central islands, corneal ectasia, decentration, and irregular astigmatism. Curr Opin Ophthalmol 2001;12:309–17. [DOI] [PubMed] [Google Scholar]
- 2.Holland SP, Srivannaboon S, Reinstein DZ. Avoiding serious corneal complications of laser assisted in situ keratomileusis and photorefractive keratectomy. Ophthalmology 2000;107:640–52. [DOI] [PubMed] [Google Scholar]
- 3.Wilson SE, Klyce SD. Screening for corneal topographic abnormalities before refractive surgery. Ophthalmology 1994;101:147–52. [DOI] [PubMed] [Google Scholar]
- 4.Neves R, Schor P, Nosé W. Ceratocone suspeito em pacientes candidatos a ceratotomia radial. Arquivos Brasileiros de Oftalmologia 1994;57:202–4. [Google Scholar]
- 5.Doyle SJ, Hynes E, Naroo S, et al. PRK in patients with a keratoconic topography picture. The concept of a physiological ‘displaced apex syndrome’. Br J Ophthalmol 1996;80:25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortensen J, Ohrstrom A. Excimer laser photorefractive keratectomy for treatment of keratoconus. J Refract Corneal Surg 1994;10:368–72. [PubMed] [Google Scholar]
- 7.Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. J Refract Surg 1995;11:371–9. [DOI] [PubMed] [Google Scholar]
- 8.Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg 1999; 25:1327–35. [DOI] [PubMed] [Google Scholar]
- 9.Smolek MK, Klyce SD. Current keratoconus detection methods compared with a neural network approach. Invest Ophthalmol Vis Sci 1997;38:2290–9. [PubMed] [Google Scholar]
- 10.El Danasoury MA, el Maghraby A, Klyce SD, et al. Comparison of photorefractive keratectomy with excimer laser in situ keratomileusis in correcting low myopia (from −2.00 to −5.50 diopters). A randomized study. Ophthalmology 1999;106:411–20; discussion 420–1. [DOI] [PubMed] [Google Scholar]
- 11.Alessio G, Boscia F, La Tegola MG, et al. Topography-driven excimer laser for the retreatment of decentralized myopic photorefractive keratectomy. Ophthalmology 2001;108:1695–703. [DOI] [PubMed] [Google Scholar]
- 12.Pop M, Payette Y. Photorefractive keratectomy versus laser in situ keratomileusis: a control-matched study. Ophthalmology 2000;107:251–7. [DOI] [PubMed] [Google Scholar]
- 13.McDonald MB, Deitz MR, Frantz JM, et al. Photorefractive keratectomy for low-to-moderate myopia and astigmatism with a small-beam, tracker-directed excimer laser. Ophthalmology 1999;106:1481–8; discussion 1488–9. [DOI] [PubMed] [Google Scholar]
- 14.Shah SI, Hersh PS. Photorefractive keratectomy for myopia with a 6-mm beam diameter. J Refract Surg 1996;12:341–6. [DOI] [PubMed] [Google Scholar]
- 15.Mannis MJ, Zadnik K, Johnson CA. The effect of penetrating keratoplasty on contrast sensitivity in keratoconus. Arch Ophthalmol 1984;102:1513–6. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Rabinowitz YS, Rotter JI, et al. Genetic epidemiological study of keratoconus: evidence for major gene determination. Am J Med Genet 2000;28:403–9. [PubMed] [Google Scholar]
- 17.Applegate RA, Hilmantel G, Howland HC, et al. Corneal first surface optical aberrations and visual performance. J Refract Surg 2000;16:507–14. [DOI] [PubMed] [Google Scholar]
- 18.Holladay JT, Dudeja DR, Chang J. Functional vision and corneal changes after laser in situ keratomileusis determined by contrast sensitivity, glare testing, and corneal topography. J Cataract Refract Surg 1999;25:663–9. [DOI] [PubMed] [Google Scholar]