Abstract
Aims: To determine the effect of the three main morphological types of cataract on refractive error.
Methods: Data were prospectively collected from 77 subjects (age 67 (SD 8) years) with one morphological type of cataract. 34 had cortical, 21 had nuclear, and 21 had posterior subcapsular cataract. 22 subjects with clear lenses (60 (7) years) were recruited as controls. The spherical equivalent and astigmatic vector change between spectacle correction and optimal refraction were calculated.
Results: The cortical cataract group showed a significant astigmatic change of 0.71 (0.67) D (mean (1 SD)) compared to the control group (0.24 (0.20) D), with 24% outside the 95% confidence limit (0.63 D). The nuclear cataract group showed a significant myopic shift of −0.38 (0.60) D compared to the control group (+0.02 (0.21) D), with 52% beyond the minus 95% confidence limit (−0.39 D).
Conclusion: A quarter of subjects with cortical cataract showed larger changes in astigmatism than subjects with clear lenses. This is probably because of the localised refractive index changes along cortical spoke opacities within the pupillary area. The well known myopic shift of nuclear cataract was also demonstrated.
Keywords: cataract, astigmatism, myopia, vector analysis
The purpose of this study was to determine the effect, if any, of the three main morphological types of age related cataract on refractive error. It is well known that nuclear cataract can cause a myopic shift in some cases.1–5 This change accounts for the “second sight of the elderly” in which the myopic shift provides normal reading ability without the need for spectacles, although distance vision worsens.3 The effect of cortical and posterior subcapsular (PSC) cataract on refractive error is less clear. Planter6 suggested that cortical opacity can induce significant hyperopic shifts, but this claim has not been repeated.7 Review papers have suggested that cortical opacity can induce astigmatic changes.3,8 However, these reports were based on clinical impression with no supporting data. Early experimental studies found no evidence that age related cataracts induce astigmatic shifts in refractive error.9,10 However, this is worth re-examining as improved methods such as vector analysis of astigmatism and better cataract grading systems should increase the sensitivity to detect these changes if they exist.
MATERIALS AND METHODS
Subjects
Subjects were recruited from the elderly patients attending for routine examination in an optometric practice in the United Kingdom between January 2001 and January 2002. The tenets of the Declaration of Helsinki were followed and the study gained approval from the university ethics committee. Informed consent was obtained from all subjects after the nature of the study had been fully explained. Inclusion criteria included age related cataract of one morphological type (patients aged over 50 years) and the ability to provide accurate responses during subjective refraction. Exclusion criteria included other ocular or systemic disease that could cause shifts in refractive error, such as diabetes mellitus, contact lens wear, Adie’s tonic pupil, idiopathic central serous chorioretinopathy or other macular oedema, orbital mass, keratoconus, eyelid mass such as chalazion, marginal corneal degenerations, pterygium, previous ocular surgery, and any systemic drug known to cause transient changes in refractive error.11 Subjects were screened using case history information, slit lamp biomicroscopy, direct and indirect ophthalmoscopy and pupil evaluation. No further selection was performed, and subjects were recruited from a consecutive series of presenting patients. Seventy seven subjects (mean age 67 (SD 8) years) were recruited who had one morphological type of cataract: 34 subjects had cortical cataract, 21 had nuclear cataract, and 21 had posterior subcapsular cataract. In addition, 22 subjects without cataract but with a similar age and fulfilling the exclusion criteria were recruited to the study to act as a control group.
Methods
Changes in refractive correction were determined by comparing the patient’s habitual correction (the refractive correction in the patient’s spectacles) to the optimal correction determined during the examination. The patient’s habitual correction was determined using lensometry (focimetry) and was recorded to the nearest 0.25DS, 0.25 DC, and 2.5 degrees. Astigmatism was recorded in the negative cylinder form. The age of the current spectacles was determined from previous records when the patient had obtained the spectacles from the optometric practice. If these were not available, the age of the spectacles were determined from the date of a previous prescription form (if available) or the patient’s recollection.
The optimal refractive correction was determined using streak retinoscopy and subjective refraction.12 The Jackson cross cylinder was used to determine astigmatism subjectively. Subjective refraction was also measured to the nearest 0.25DS, 0.25 DC, and 2.5 degrees. Spherical changes in refractive correction were calculated from the spherical equivalent value (sphere + ½ cylinder). Astigmatic changes were determined by vector analysis,13 which takes into account both the magnitude and the direction of two cylinders when calculating their difference.
Cataracts were classified with the LOCS III system using the slit lamp biomicroscope after pupil dilatation with 0.5% or 1% tropicamide.14 There is no clear cut-off point at which normal ageing changes in the lens end and cataract begins. For the purposes of this study, nuclear or cortical opacities less than LOCS 2.0 were regarded as normal, ageing changes15: for example, a subject with opacity graded as nuclear 3.2, cortical 1.8 was regarded as having nuclear cataract only. Any posterior subcapsular opacity was regarded as cataract, as it can be very visually disabling even in its early stages.16 The location of all PSC and cortical cataracts was drawn, to investigate if any relation existed between opacity location and axis of astigmatism.
Statistics
Refraction data were stored in a spreadsheet and converted into spherical equivalents and vectors for calculation of spherical and astigmatic changes respectively. The differences in mean sphere are shown as positive if the optimal refractive correction was more hyperopic (less myopic) than the patient’s spectacles and negative if the optimal refractive correction was more myopic (less hyperopic) than the patient’s spectacles. The vector analysis of astigmatic change was performed using the Alpins method.13 We have substituted the term cataract induced astigmatism (CIA) as an analogy for Alpins’ surgical induced astigmatism (SIA)17 so called because the Alpins method is commonly used to describe astigmatic change after refractive surgery. Cataract induced astigmatism is recorded as positive. The differences between cataract morphology groups for age, age of spectacles, spherical refractive error shift, and astigmatic changes in refraction (CIA) were determined with analysis of variance (ANOVA) with post hoc significance testing (Scheffé F test). All statistical analyses were performed on SPSS for windows (SPSS Inc).
RESULTS
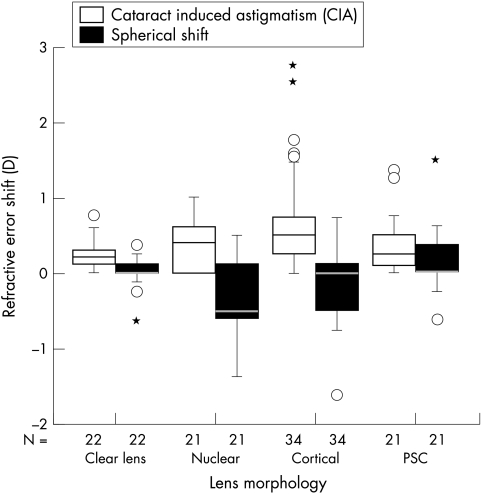
The mean and 1 SD data for each subject group of age, age of current spectacles, spherical refractive shift, and astigmatic shift are shown in Table 1. ANOVA indicated a significant difference in the ages of the four groups (F3,94,= 11.6, p <0.0001), which post hoc analysis using the Scheffé F test indicated was because of the control group was significantly younger than the three groups with cataract (p<0.05). Similarly, post hoc analysis indicated that the four groups had similar spectacle ages, except for the nuclear and PSC cataract groups (p<0.05). The spherical and astigmatic refractive error shifts are also shown in Figure 1 for each morphological cataract group.
Table 1.
Mean (±1 SD) age, age of spectacles, and spherical and astigmatic refractive error shift for each subject group
| No | Subject age (years) | Age of spectacles (months) | Spherical refractive error shift (DS) | Astigmatic refractive error shift (D) | |
| Control group | 22 | 60 (7) | 33 (19) | +0.02 (0.21) | 0.24 (0.20) |
| Nuclear cataract | 21 | 70 (8) | 44 (15) | −0.38 (0.60) | 0.38 (0.31) |
| Cortical cataract | 34 | 71 (9) | 34 (9) | −0.18 (0.46) | 0.71 (0.67) |
| PSC cataract | 21 | 66 (5) | 25 (14) | +0.15 (0.43) | 0.35 (0.38) |
Figure 1.
Spherical refractive error shift and cataract induced astigmatism for each lens morphology. The nuclear cataract group shows a more pronounced myopic shift and the cortical cataract group shows more astigmatic change. The box represents the middle half of the ranked data and outliers appear as individual cases beyond the 95% confidence limits.
The changes in the spherical refractive error were significantly different in the four groups (ANOVA, F3,94= 5.9, p = 0.001). Post hoc analysis using the Scheffé F test indicated that this was due to differences between the nuclear cataract group and the clear lens and PSC groups (p<0.05). The control group showed a mean (1 SD) spherical refractive shift of +0.02 (0.21) D, so that the more myopic 95% confidence limit (mean −1.96 SD) for the control group was −0.39 D. Subjects with nuclear cataract and a minus shift greater than −0.39 D were deemed to have a significant myopic shift. This occurred for 11/21 (52%) of the subjects with nuclear cataract. A significant minus shift was also found for 10/34 (29%) of the subjects with cortical cataract and one subject (5%) with PSC cataract.
The changes in astigmatic refractive correction were significantly different in the four groups (ANOVA, F3,94= 5.4, p = 0.0018). Post hoc analysis using the Scheffé F test indicated that this was the result of differences between the cortical cataract group and the clear lens group (p<0.05). The control group showed a mean (1 SD) astigmatic refractive shift of +0.24 (0.20), so that the upper 95% confidence limit (mean (SD 1.96)) for the control group was 0.63. Subjects with cortical cataract with an astigmatic shift greater than 0.63 were deemed to have significant induced astigmatism. This occurred for 8/34 (24%) of the subjects with cortical cataract. A significant astigmatic shift was also found for three subjects (14%) with nuclear cataract and three subjects (14%) with PSC cataract.
Visual inspection of the lens drawings in the eight subjects with cortical cataract suggested that the cataracts that produced large astigmatic changes were those with a single spoke that entered the undilated pupil area. A typical case is presented in Figure 2, which shows a horizontal cortical spoke in a subject who was refracted 31 months before at +0.25/−0.50 × 70, but refracted to 0.00/−1.25 × 10 6/9. The small number of cases with a significant astigmatic shift meant we were unable to statistically test this hypothesis.
Figure 2.
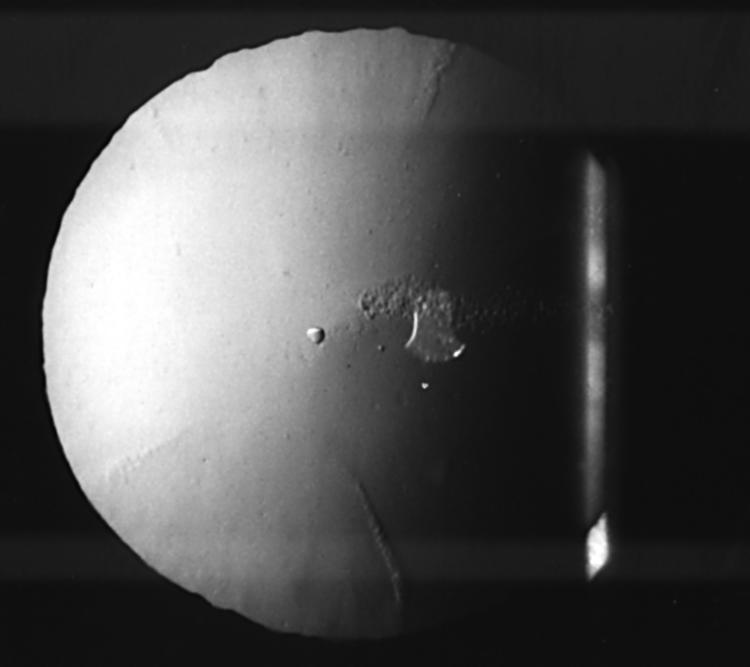
Retroillumination view of the lens showing a horizontal cortical spoke in a patient whose refractive error changed from +0.25/−0.50 × 70 to 0.00/−1.25 × 10—that is, an astigmatic shift of −1.56 × 2.
DISCUSSION
Posterior subcapsular cataract showed refractive changes similar to the age matched control group with clear lenses, so their data warrants no further discussion. As previously reported,1–5 nuclear cataract can cause significant myopic refractive error shifts. This is probably caused by symmetrical refractive index changes within the nucleus of the lens, causing negative spherical aberration and a myopic shift.18,19 In our small sample, this occurred in approximately half the subjects with nuclear cataract. A more accurate figure for the prevalence of myopic shifts in patients with nuclear cataract could be obtained with a larger sample. We suggest that the slight myopic shift seen in some of the subjects with cortical cataract is more probably the result of early nuclear opacity (LOCS III NO grade less than 2.0 defined as a clear lens in this study) rather than the cortical opacity itself. The majority of cataracts are of mixed type and it is difficult to find cataracts of just one morphological type.1 As mentioned earlier, it is difficult to determine when age related loss of transparency ends and cataract begins, so the LOCS III grade of 2.0 is somewhat arbitrary. Furthermore, Brown has previously stated that a myopic shift may precede the onset of visible nuclear cataract.1 Other studies on very early cataracts have shown that although nuclear cataracts show negative spherical aberration (and thus a minus shift in refractive error), this is not the case with cortical cataracts, which tend to show slight positive spherical aberration that is similar to control subjects with clear lenses.19 We acknowledge that this study is limited by not being able to exclude axial elongation as a cause of myopia; however, myopic shift with nuclear sclerosis is an accepted phenomenon whereas axial myopia in the elderly is not, and no myopic shift occurred in the PSC or control group.
We found that cortical cataract can cause significant astigmatic shifts. In our small sample, this occurred in about a quarter of subjects with cortical cataract. Previous studies that have looked for astigmatic shifts in patients with cataract have examined the lens using an undilated pupil and did not use any formal categorisation of cataract.9,10 In addition, they were unable to integrate cylindrical power and axis changes as we have done with modern statistical methods, so that analyses were somewhat rudimentary. Wheelock9 hypothesised that only against the rule astigmatism could be caused by cataract and compared the prevalence of such changes in cataract and control subjects. Lyle10 included all morphological types of cataract in his analyses, so that any astigmatic shift (according to our results likely to be found in a small percentage of those with cortical cataract) appears to have been lost in these data. We assume that the astigmatic shift is due to cataract induced astigmatism (CIA) rather than to corneal changes. Although it is well known that corneal astigmatism changes with age from with the rule to against the rule, this is a slow process and some of the cortical cataract subjects show significantly more astigmatic shift than the control group. In addition, the age of the spectacles of the subjects with cortical opacity was no older than those from the other groups. Ideally, one could take longitudinal keratometer measurements of corneal shape in addition to spectacle refractive error changes to rule out possible astigmatic changes in subjects with cortical opacity due to corneal shape. However, these were taken for one subject who showed no change in keratometer measurements, despite a significant astigmatic change. Moreover, Kusoda and colleagues19 showed no change in corneal higher order aberrations in subjects with cortical (and nuclear) cataract, despite changes in cataract induced aberrations. The changes in astigmatism were significantly more in the cortical group than in the nuclear, PSC, or control group. It seems unlikely that significant changes in corneal shape in subjects with cortical opacity would be responsible for this phenomenon.
The mechanism by which cortical cataract induces astigmatism is unclear. It is unlikely to be due to lens curvature changes as this would require the cortical cataract to cause a change in lens thickness that has previously been shown to not occur.20 The CIA is more likely to be caused by asymmetrical refractive index changes within parts of the cortex of the lens,21 causing coma-like aberration and astigmatic shifts in refractive error.18 The suggestion that the negative axis of astigmatism may correspond to the axis of a cortical spoke in the undilated pupil supports this hypothesis. In our small sample, a significant astigmatic shift occurred in about a quarter of the subjects with cortical cataract. A more accurate figure for the prevalence of astigmatic shifts in patients with cortical cataract could be obtained with a larger sample. We suggest that the slight CIA seen in some of the subjects with nuclear cataract is more probably the result of early cortical opacity (LOCS III C grade less than 2.0 defined as a clear lens in this study) rather than the nuclear opacity itself.
CONCLUSION
This study suggests that approximately a half of patients with nuclear cataract have a significant myopic shift and a quarter of patients with cortical cataract have a significant astigmatic shift. Given the high prevalence of age related cataract, the refractive error induced by nuclear and cortical cataract is likely to be a major cause of the uncorrected refractive error in the elderly.22,23 This suggests that patients who have early nuclear and/or cortical cataract should have their refractive error assessed on a regular basis. This finding could also be important in the interpretation of epidemiological studies that have attempted to determine whether refractive error is a risk factor for cataract as results would be confounded if refractive error shifts were caused by cataract.24,25
Acknowledgments
Presented in part at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, Fort Lauderdale, Florida, USA on 6 May 2002.
Konrad Pesudovs is supported by National Health and Medical Research Council (NHMRC, Canberra, Australian Capital Territory, Australia) Sir Neil Hamilton Fairley Fellowship 007161.
Neither author has any commercial or proprietary interest or association in any product or technique used in this study.
REFERENCES
- 1.Brown NAP, Hill AR. Cataract, the relation between myopia and cataract morphology. Br J Ophthalmol 1987;71:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin ML. Opalescent nuclear cataract. J Cataract Refract Surg 1989;15:576–9. [DOI] [PubMed] [Google Scholar]
- 3.Brown NA. The morphology of cataract and visual performance. Eye 1993;7:63–7. [DOI] [PubMed] [Google Scholar]
- 4.Wensor M, McCarty CA, Taylor HR. Prevalence and risk factors of myopia in Victoria, Australia. Arch Ophthalmol 1999;117:658–63. [DOI] [PubMed] [Google Scholar]
- 5.Reeves BC, Hill AR, Brown NA. Myopia and cataract. Lancet 1987;2:964. [DOI] [PubMed] [Google Scholar]
- 6.Planten JT, B de Vries AK, Woldringh JJH. Pathological approach of cataract and lens. Ophthalmologica 1978;176:331–4. [DOI] [PubMed] [Google Scholar]
- 7.Rouhiainen P, Rouhiainen H, Salonen JT. The impact of early lens opacity progression on visual acuity and refraction. Ophthalmologica 1997;211:242–6. [DOI] [PubMed] [Google Scholar]
- 8.Elliott DB. Evaluating visual function in cataract. Optom Vis Sci 1993;70:896–902. [DOI] [PubMed] [Google Scholar]
- 9.Wheelock A. Cataract and against-the-rule astigmatism. Am J Optom Arch Am Acad Optom 1941;18:489–92. [Google Scholar]
- 10.Lyle W. Changes in astigmatism associated with the development of cataract. Am J Optom Arch Am Acad Optom 1951;28:551–9. [DOI] [PubMed] [Google Scholar]
- 11.Locke L. Induced refractive and visual changes. In: Amos J, ed. Diagnosis and managment in vision care. Boston: Butterworths, 1987:313–67.
- 12.Elliott DB. Clinical procedures in primary eye care. Oxford: Butterworth-Heinemann, 1997.
- 13.Alpins NA. Astigmatism analysis by the Alpins method. J Cataract Refract Surg 2001;27:31–49. [DOI] [PubMed] [Google Scholar]
- 14.Chylack LT Jr, Wolfe JK, Singer DM, et al. The lens opacities classification system III. Arch Ophthalmol 1993;111:831–6. [DOI] [PubMed] [Google Scholar]
- 15.Pesudovs K, Coster DJ. Assessment of visual function in cataract patients with a mean visual acuity of 6/9. Aust N Z J Ophthalmol 1996;24:5–9. [DOI] [PubMed] [Google Scholar]
- 16.Adamsons I, Rubin GS, Vitale S, et al. The effect of early cataracts on glare and contrast sensitivity. A pilot study. Arch Ophthalmol 1992;110:1081–6. [DOI] [PubMed] [Google Scholar]
- 17.Alpins NA. New method of targeting vectors to treat astigmatism. J Cataract Refract Surg 1997;23:65–75. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda T, Fujikado T, Maeda N, et al. Wavefront analysis of higher-order aberrations in patients with cataract. J Cataract Refract Surg 2002;28:438–44. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda T, Fujikado T, Maeda N, et al. Wavefront analysis in eyes with nuclear or cortical cataract. Am J Ophthalmol 2002;134:1–9. [DOI] [PubMed] [Google Scholar]
- 20.Laursen AB, Fledelius H. Variations of lens thickness in relation to biomicroscopic types of human senile cataract. Acta Ophthalmol (Copenh) 1979;57:1–13. [DOI] [PubMed] [Google Scholar]
- 21.Planten JT. Changes of refraction in the adult eye due to changing refractive indices of the layers of the lens. Ophthalmologica 1981;183:86–90. [DOI] [PubMed] [Google Scholar]
- 22.Wormald RP, Wright LA, Courtney P, et al. Visual problems in the elderly population and implications for services. BMJ 1992;304:1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haegerstrom-Portnoy G, Schneck ME, Brabyn JA, et al. Development of refractive errors into old age. Optom Vis Sci 2002;79:643–9. [DOI] [PubMed] [Google Scholar]
- 24.McCarty CA, Mukesh BN, Fu CL, et al. The epidemiology of cataract in Australia. Am J Ophthalmol 1999;128:446–65. [DOI] [PubMed] [Google Scholar]
- 25.Wong TY, Klein BE, Klein R, et al. Refractive errors and incident cataracts:the beaver dam eye study. Invest Ophthalmol Vis Sci 2001;42:1449–54. [PubMed] [Google Scholar]