Abstract
Aim: The use of an animal model to study the aqueous dynamic and the histological findings after deep sclerectomy with (DSCI) and without collagen implant.
Methods: Deep sclerectomy was performed on rabbits’ eyes. Eyes were randomly assigned to receive collagen implants. Measurements of intraocular pressure (IOP) and aqueous outflow facility using the constant pressure method through cannulation of the anterior chamber were performed. The system was filled with BSS and cationised ferritin. Histological assessment of the operative site was performed. Sections were stained with haematoxylin and eosin and with Prussian blue. Aqueous drainage vessels were identified by the reaction between ferritin and Prussian blue. All eyes were coded so that the investigator was blind to the type of surgery until the evaluation was completed.
Results: A significant decrease in IOP (p<0.05) was observed during the first 6 weeks after DSCI (mean IOP was 13.07 (2.95) mm Hg preoperatively and 9.08 (2.25) mm Hg at 6 weeks); DS without collagen implant revealed a significant decrease in IOP at weeks 4 and 8 after surgery (mean IOP 12.57 (3.52) mm Hg preoperatively, 9.45 (3.38) mm Hg at 4 weeks, and 9.22 (3.39) mm Hg at 8 weeks). Outflow facility was significantly increased throughout the 9 months of follow up in both DSCI and DS groups (p<0.05). The preoperative outflow facility (OF) was 0.15 (0.02) μl/min/mm Hg. At 9 months, OF was 0.52 (0.28) μl/min/mm Hg and 0.46 (0.07) μl/min/mm Hg for DSCI and DS respectively. Light microscopy studies showed the appearance of new aqueous drainage vessels in the sclera adjacent to the dissection site in DSCI and DS and the apparition of spindle cells lining the collagen implant in DSCI after 2 months.
Conclusion: A significant IOP decrease was observed during the first weeks after DSCI and DS. DS with or without collagen implant provided a significant increase in outflow facility throughout the 9 months of follow up. This might be partly explained by new drainage vessels in the sclera surrounding the operated site. Microscopic studies revealed the appearance of spindle cells lining the collagen implant in DSCI after 2 months.
Keywords: deep sclerectomy, rabbit, collagen glaucoma drainage device, glaucoma surgery
In an attempt to increase the success rates of non-penetrating glaucoma surgery, Kozlov et al1 described using a collagen implant in order to bridge the period of maximal wound healing postoperatively.
Although deep sclerectomy with collagen implant (DSCI) is now a recognised procedure,2–8 performed successfully in several ophthalmic centres, there has been until now no animal model of this surgery.
The aim of our study was thus to describe an animal model of deep sclerectomy, and to study the mechanisms of filtration, the aqueous humour dynamics, the evolution of the collagen implant with time, and the scarring response of the different tissues after deep sclerectomy.
STUDY DESIGN
Deep sclerectomies were performed in both eyes of six groups of three rabbits. The left and right eyes were randomly assigned to undergo either DSCI or deep sclerectomy without collagen implant (DS).
The follow up of the different groups varied from 2 weeks to 9 months. During this time, regular intraocular pressure (IOP) measurements were performed (2 weeks after surgery and then once a month) with a Tono-Pen XL (Mentor, Santa Barbara, CA, USA) tonometer under local anaesthesia and complete ocular examinations were performed.
Rabbits were killed in groups, each group including three rabbits, at 2 weeks and at 1, 2, 3, 6, and 9 months postoperatively. Before killing the animal, outflow facility (OF) measurements of both eyes were performed using the constant pressure method through cannulation of the anterior chamber.
To study the site of aqueous filtration histologically, ferritin was injected into the anterior chamber during the cannulation. Eyes were then enucleated and fixed for light microscopy.
MATERIALS AND METHOD
Our experiments have been performed on pigmented female rabbits weighing 2.0–3.0 kg. Animals were kept at 21°C in a normal 12 hours light and 12 hours dark cycle. All the rabbits were kept individually in separate cages.
For the experiments (surgery, secondary flow studies, and enucleation), all animals were anaesthetised by intramuscular injection of 1–4 ml/kg of a solution of 5 ml ketamine (100 mg/ml) and 5 ml xylazine (20 mg/ml). At the end of the experiment and while still under anaesthesia, the animals were killed by intracardiac injection of 3–5 ml of a solution of sodium pentobarbital (65 mg/ml).
Experiments have been performed in compliance with the ARVO statement for the use of animals in ophthalmic research and with the cantonal veterinary service of Lausanne, Switzerland.
Surgical procedure
The same surgeon (AM) performed all the DSCI and DS. To expose the surgical site an intracorneal 6-0 silk traction suture was first placed on the limbal cornea. The conjunctiva was then opened at the limbus in the upper part of the eye and the sclera was exposed. A limbal based scleral flap measuring 5×5 mm was dissected using a diamond knife. The deep sclerectomy was then performed leaving a thin layer of deep sclera over the choroid and the ciliary body; Schlemm’s canal was deroofed. At this stage of the surgery, a significant aqueous humour filtration through the remaining trabeculum was observed.
If the eye was randomly selected to receive a collagen implant (CGDD-10 implant, Starr Surgical AG, Nidau, Switzerland), the device measuring 1×1×4 mm was sutured radially in the scleral bed with a 10/0 nylon suture. The superficial scleral flap was repositioned over the collagen implant and secured with two loose 10/0 nylon sutures. If the eye was randomly selected not to receive a collagen implant, the superficial scleral flap was closed with two 10/0 nylon sutures after the deep scleral dissection. In all eyes, the conjunctiva was closed with interrupted 8/0 vicryl sutures. After the intervention, topical application of tobramycin and dexamethasone (Tobradex, Alcon, Forth Worth, TX, USA) was given twice a day for 3 weeks.
Outflow facility measurement9,10
Under microscopic control, the anterior chamber of the eyes was cannulated at the limbus using a 30 gauge needle. The puncture site was watertight; the tip of the needle was positioned between the anterior iris plane and the cornea. The needle was then connected via a polyethylene tubing to a microsyringe pump (SP 200i, World Precision Instruments, Inc, Sarasota, FL, USA) which allows various flow rates ranging from 0.2 μl/hour to 426 ml/hour. The needle syringe system was also connected via polyethylene tubings to a water manometer and an electronic pressure transducer (Type BLPR, World Precision Instruments, Inc, Sarasota, FL, USA). Pressure measurements were then amplified with an amplifier and displayed on a pressure monitor (Ape BP-1, World Precision Instruments, Sarasota, FL, USA) and printed using a chart recorder (type L200E, Linseis GmbH, Selb Germany).
The intraocular pressure could be increased or lowered by adjusting the height of the water manometer. The water reservoir of the water manometer was also used to fill the tube and flush air bubbles. The intraocular pressure was measured in mm Hg on the chart recorder, which, at the beginning of the measurements, was calibrated against the column of water.
The system was filled with BSS (Alcon, Forth Worth, TX, USA) and cationised ferritin (ferritin from horse spleen, 50 mg/ml, PM = 800 000, Biochemika, Fluka Chemie, Buchs, Switzerland), so that ferritin entered the anterior chamber of the eye right from the start of outflow facility measurements.
After cannulation of the eye, the pressure system was adjusted at the lowest pressure at which a flow was detectable. IOP was then increased in 10 mm Hg increments up to a maximum of 60 mm Hg. At each pressure level, the infusion flow was precisely adjusted to maintain a constant IOP. This adjustment was done by varying the infusion flow; increasing the flow when IOP was decreasing and vice versa. During the adjustment period, the system was reduced to a closed system (eye-syringe-pressure transducer). The closed system was completely waterproof. Therefore the solution that was infused with the pump to maintain a stable IOP, corresponded to the outflow of the eye minus the aqueous produced by the ciliary body.
The infusion flow measurements were then plotted against the pressures in the system and a regression line was obtained. The slope of the regression line represents the outflow facility (μl/min/mm Hg).
The outflow facility (C) was then calculated using the Goldmann equation11:
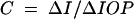 |
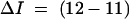 |
where I1 and I2 are successive inflow rates (μl/min)
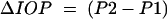 |
where P1 and P2 represent IOP at I1 and I2 respectively (mm Hg)
IOP measurement
Preoperatively, 2 weeks and then once a month postoperatively, IOP measurements were performed with a Tono-Pen tonometer under local anaesthesia (Novesine, Ciba Vision, Bülach, Switzerland). The mean of five consecutive readings was recorded.
Mean outflow facility of normal rabbit (unoperated rabbit)
Measurements of preoperative outflow facility were performed on five fresh enucleated eyes, with the same equipment used as for outflow facility studies mentioned above. The average of these five measurements defined the preoperative outflow facility.
Morphometric analysis
The rabbits’ eyes were enucleated just after the animals were killed and fixed in formalin for paraffin embedding. Sections were stained with haematoxylin and eosin (H&E) and some with Prussian blue, which reacts with ferritin. All eyes were coded so that the investigator was blind to the type of surgery until the evaluation was completed. Histological assessment was done to assess the route of aqueous humour from the anterior chamber to the subconjunctival bleb, uveoscleral tissues, and physiological outflow pathways.
Structure, size, and surrounding tissue reactions (trabeculo-Descemets membrane, scleral bed, scleral flap, Schlemm’s canal, filtration bleb and choroidal space) were assessed. To determine the amount of aqueous humour resorption inside the scleral tissues surrounding the site of surgery, the number of drainage vessels adjacent to the dissection were recorded using light microscopy: 10 consecutive sections of each eye were observed with the same magnification (×100). The aqueous drainage vessels were identified by their blue colour caused by the reaction between ferritin and Prussian blue. For each eye in DS and DSCI procedures, the number of vessels in 10 consecutive sections was recorded first at the surgical site and then 180° opposite to the surgical site where the tissues were untouched. The results between these two sites were then compared.
RESULTS
During the DSCI and DS surgical procedures, no perforation of the trabecular membrane occurred. In the follow up period, one rabbit in the 6 month follow up group died at 4 months after the operation for unknown reasons and was therefore excluded from analysis. The IOP measurements were performed on both eyes under topical anaesthesia: this manoeuvre was easy and well tolerated by animals throughout the study. No eyes presented postoperative inflammation in the anterior chamber. All corneas stayed clear and no endophthalmitis was observed.
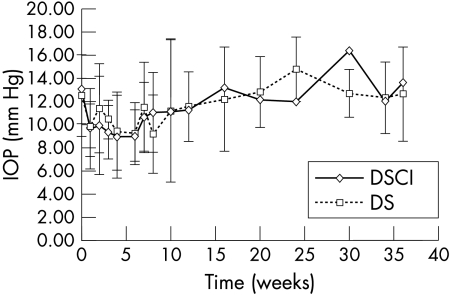
Comparison between mean IOP after DS with and without implant is represented in Figure 1: with both types of surgery an initial IOP decrease was observed for 6–8 weeks postoperatively. The IOP decrease was statistically significant during the 6 weeks after DSCI (mean preoperative IOP: 13.07 (SD 2.95) mm Hg; mean postoperative IOP (6w): 9.08 (2.25) mm Hg; p = 0.009). The IOP decrease was statistically significant after DS at weeks 4 and 8 (mean preoperative IOP: 12.57 (3.52) mm Hg; mean postoperative IOP (4w): 9.45 (3.38) mm Hg, p = 0.036; mean postoperative IOP (8w): 9.22 (3.39) mm Hg, p = 0.035). After the initial decrease, the mean IOP returned to the preoperative value after 16 weeks for DS and DSCI.
Figure 1.
Comparison of mean intraocular pressure (IOP) after deep sclerotomy with (DSCI) and without (DS) collagen implant during the 9 months of follow up. After an initial significant decrease, the mean IOP returns to preoperative value after 21 months.
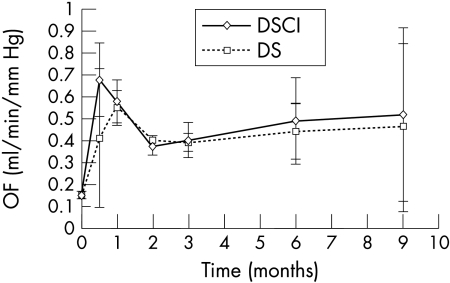
Comparison between preoperative and postoperative mean outflow facilities is represented in Figure 2. A significant increase in outflow facility was observed during the entire 9 months of follow up in both DSCI and DS groups (p<0.05); the mean preoperative outflow facility was 0.15 μl/min/mm Hg; at 9 months, outflow facility was 0.52 (0.28) μl/min/mm Hg (p = 0.012) and 0.46 (0.07) μl/min/mm Hg (p<0.001) for DSCI and DS respectively. The slope of the mean outflow facility during the study was similar in both groups: initially after surgery (first 2 months), the mean outflow facility in both DS and DSCI showed a peak after which it decreased before being stabilised.
Figure 2.
Comparison of preoperative and postoperative mean outflow facility (OF) in DSCI and DS. A significant increase in OF was observed during the entire 9 months of follow up in both DSCI and DS.
The outflow facility after 2 weeks was higher in the DSCI group than in the DS group, but this difference was not statistically significant.
Light microscopy studies (Figs 3–9)
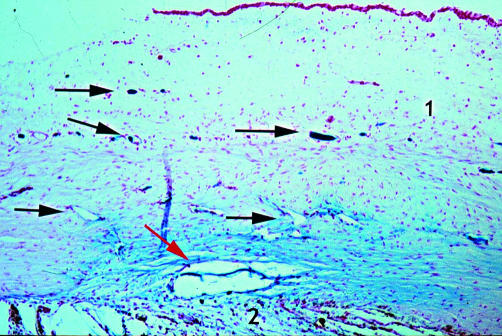
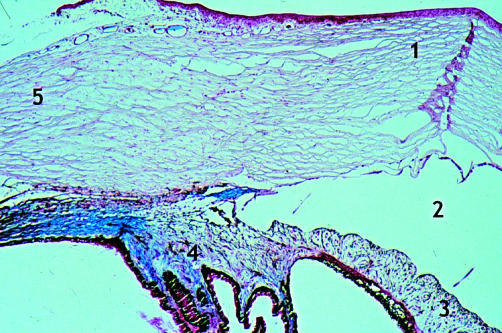
Figure 3.
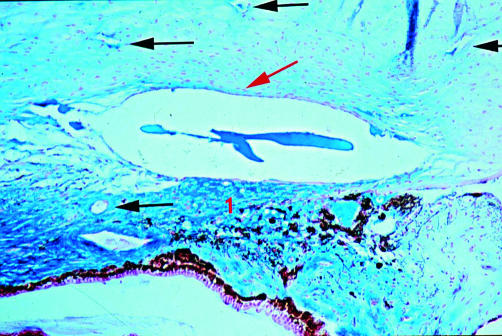
Rabbit 11; 2 months post DSCI (×25). A few spindle cells are seen lining the canal walls (red arrow). New aqueous drainage vessels easily identified by their blue colour (arrow). 1, sclera; 2, ciliary body.
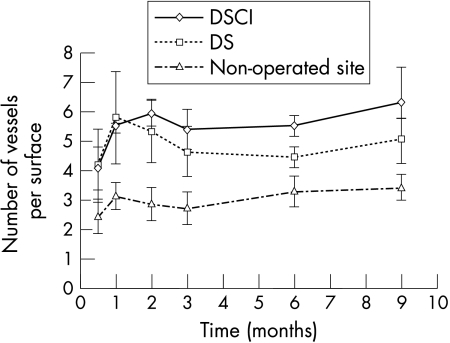
Figure 9.
Number of aqueous drainage vessels in the scleral filtration space after DSCI and DS and in the unoperated site. The number of new drainage vessels in the operated sclera with or without collagen implant is significantly higher than the number of drainage vessels recorded in the unoperated sclera.
In both DSCI and DS groups, a drainage canal was observed from 2 weeks up to the 9 months of follow up. The main difference between DSCI and DS sections was the presence of spindle cells lining the walls of this canal in DSCI sections: these spindle cells were sparsely present 2 months after DSCI (rabbit 11 only; Fig 3). Six months postoperatively, they covered the entire lining of the canal (Fig 4). In comparison, in DS sections, an irregular canal without spindle cells was observed (Figs 5 and 6).
Figure 4.
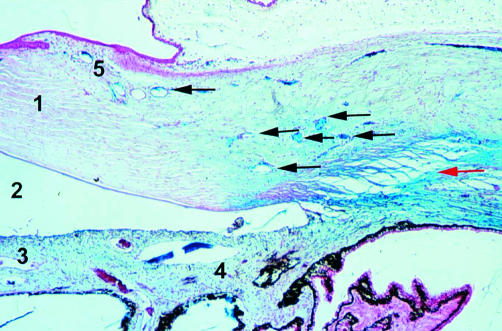
Rabbit 3; 9 months post-DSCI (×50). Spindle cells cover the entire wall of the canal (red arrow). New aqueous drainage vessels surrounding the operated site are present (arrow). 1, Ciliary body.
Figure 5.
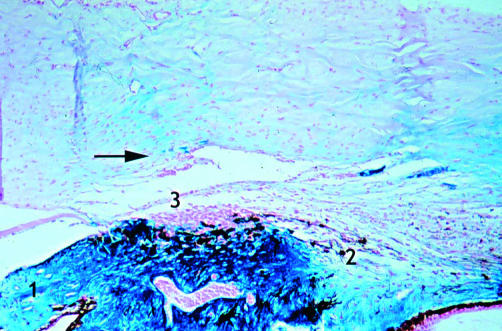
Rabbit 14; 1 month post-DS (×10). Irregular canal at the operated site without spindle cells (red arrow). Appearance of aqueous drainage vessels in the sclera adjacent to the dissection site (blue coloration caused by the reaction between ferritin and Prussian blue dye) (arrow). 1, Cornea; 2, anterior chamber; 3, iris; 4, ciliary body; 5, aqueous veins.
Figure 6.
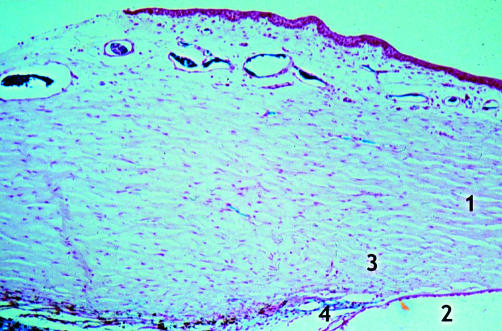
Rabbit 3; 9 months post-DS (×25). Irregular canal at the operative site (red arrow). 1, Iris; 2, ciliary body; 3, trabeculum.
Microscopic studies revealed the appearance of new drainage vessels in the sclera adjacent to the dissection site in both surgical groups (Figs 7 and 5; Figs 3 and 8). The number of new aqueous drainage vessels in the operated sclera with or without collagen implant (Figs 5 and 6, respectively) compared with the unoperated sclera (Figs 7 and 8) was significantly higher during the 9 months of follow up. The number of these vessels was less in DS than in DSCI, but this difference was not statistically significant (Fig 9). No inflammatory reactions were observed at any time after surgery (DSCI and DS).
Figure 7.
Rabbit 11; unoperated site (×25). Comparison with Figure 3.1, Cornea; 2, anterior chamber; 3, Schlemm’s canal; 4, trabeculum.
Figure 8.
Rabbit 14; unoperated site (×10). Comparison with Figure 5. 1, Cornea; 2, anterior chamber; 3, iris; 4, ciliary body; 5, sclera.
DISCUSSION
The main advantage of deep sclerectomy, with or without collagen implant, is the relatively low complication rate associated with it.2–5,12,13 In our series, no postoperative complications were observed after DS and DSCI. There was no hyphaema, no flat or shallow anterior chamber, no anterior chamber inflammation and no surgically induced cataract. The rabbit appears to be an appropriate animal model for the study of non-penetrating filtering surgery.
After DSCI a significant IOP, decrease was observed during the first six postoperative weeks; whereas after DS it was only significant at weeks 4 and 8. The lowest pressures measured were 9 mm Hg in DSCI group at 4 weeks and 9.2 mm Hg in the DS group at 8 weeks. At 24 and 30 weeks after surgery (DS and DSCI respectively), we observed a statistically non-significant transient rise in IOP, which could reflect the activity of the scarring process.
To assess the preoperative outflow facility, we measured the outflow facility of five freshly enucleated eyes of unoperated rabbits. The average of these five measurements provided a preoperative outflow facility of 0.15 (0.02) μl/min/mm Hg. The postoperative outflow facility in both the DSCI and DS groups was significantly increased for the entire 9 months of follow up (p<0.05). However a drop in outflow facility was noted after the first 2 weeks in both groups and this might be, partly at least, explained by the scarring process. These results show that, at least in the first few months after surgery, there is no real benefit in terms of outflow facility of adding a collagen implant to the DS procedure. However, in the human study by Sanchez et al,2 the collagen implant after 2 months was shown to be beneficial. A longer follow up in this study would thus be of interest.
There is then a discrepancy between the significant increase in outflow facility, which was evident during the entire study, and the rise of IOP to preoperative values after 2 months. We could not find any explanation for this phenomenon; probably a study with a greater number of rabbits would be able to offer an appropriate explanation.
One possible explanation relates to the technical aspects of outflow facility determination. The IOP is increased to various levels and the infusion flow is determined for those levels. The slope of the regression line for the IOP versus flow represents the outflow facility. This calculation is correct for a non-surgical eye, but may not specifically apply after certain surgeries. Specifically, the outflow facility may be high at higher IOPs but not at lower (more physiological) IOPs, acting almost as a variable resistor or valve.
The collagen implant used in DSCI dissolves in the conventional medium used for the preparation of sections (Xylol). This was demonstrated by fixing the implant in formalin, followed by paraffin embedding. Under light microscopy, an “open space” or a “canal” inside the sclera was seen, which remained after implants’ resorption. It is also not possible with this method of section preparation to evaluate the resorption time of the collagen device. Furthermore there was no relation between the size of the canal and the time relapsed after DSCI.
Canals were also noted in the DS group and probably resulted from dissection only in this type of surgery. However, these canals of filtration were very different depending on the surgical procedure. In DSCI, a few spindle cells lining the canal started to appear after 2 months. At 6 and 9 months, the entire walls were covered with a thin layer of spindle cells. In DS as well, a canal resulting from the dissection could be observed until 9 months. However, cells lining the canal walls were never found.
One can possibly conclude from this observation that the use of collagen implant induces the lining of the resulting canal by spindle cells. Whether this phenomenon offers relative advantages, possibly keeping this canal patent for extended periods, remains to be seen. Further studies with longer follow ups would certainly be needed to delineate or disprove such a theory.
Another interesting finding was the presence of new aqueous vessels in the sclera adjacent to the dissection site in both DSCI and DS groups. Ferritin injected at the beginning of the outflow facility measurements follows the aqueous route. By reacting with Prussian blue dye it colours these vessels blue, which are easily seen under light microscopy; in contrast, blood vessels do not stain. The number of new aqueous vessels in the operated sclera with or without collagen implant compared with the unoperated sclera was significantly higher, independent of the postoperative time. This difference was slightly less pronounced in DS but it was not statistically significant.
The increase in outflow facility, observed during the first 2 months postoperatively in DSCI and DS, corresponded with the period where the vessels were the more frequent in the sclera. The increase in sclera, which follows the surgery, seemed then to lead to new drainage vessels.
No inflammatory reaction apart from the one occasionally related to a nylon suture was ever observed.
CONCLUSION
A significant IOP decrease was observed during the 2 months following DSCI and DS.
DS with or without collagen implant provided a significant increase in outflow facility for at least 9 months. However, it was not possible to conclude after this study whether the collagen implant would allow better results in the long term.
Microscopic studies revealed the persistence of a drainage space for up to 9 months in both DSCI and DS sections: the canals were irregular in DS, whereas in DSCI spindle cells were present as early as 2 months after surgery and at 9 months the entire canal was covered with these cells. The collagen implant was well tolerated by rabbit tissues, as no inflammatory responses were observed during the entire study. The long term increase in outflow facility might be partly explained by new drainage vessels surrounding the operated site.
Finally, the authors acknowledge that the sclera and Tenon capsule’s reaction may be very different in the human situation and cadaver specimens will be helpful to substantiate the findings.
Financial interest: The study was sponsored by a research grant of STAAR Surgical AG, Nidau, Switzerland.
REFERENCES
- 1.Fyodorov SN, Kozlov VI. Non penetrating deep sclerectomy in open angle glaucoma. Eye Microsurgery (in Russian) 1989:52–5.
- 2.Sanchez E, Schnyder CC, Sickenberg M, et al. Deep sclerectomy: results with and without collagen implant. Int Ophthalmol 1997;20:157–62. [DOI] [PubMed] [Google Scholar]
- 3.Kozlov VI, Bagrov SN, Anisimova SY, et al. Non penetrating deep sclerectomy with collagen. Eye Microsurgery (in Russian) 1990;3:44–6. [Google Scholar]
- 4.Chiou AGY, Mermoud A, Hediguer SE, et al. Ultrasound biomicroscopy of eyes undergoing deep sclerectomy with collagen implant. Br J Ophthalmol 1996;80:541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiou AGY, Schnyder CC, Mermoud A, et al. An ultrasound biomicroscopic study of eyes after deep sclerectomy with collagen implant. Ophthalmology 1998;105:746–50. [DOI] [PubMed] [Google Scholar]
- 6.Demailly P, Jeanteur-Lunel MN, Berkani M, et al. Non penetrating deep sclerectomy associated with collagen device in primary open angle glaucoma. Middle-term retrospective study. J Fr Ophtalmol 1996;11:659–66. [PubMed] [Google Scholar]
- 7.Karlen ME, Sanchez E, Schnyder CC, et al. Deep sclerectomy with collagen implant: medium term results. Br J Ophthalmol 1999;83:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mermoud A, Schnyder CC, Sickenberg M, et al. Comparison of deep sclerectomy with collagen implant and trabeculectomy in open-angle glaucoma. J Cataract Refract Surg 1999;25:323–31. [DOI] [PubMed] [Google Scholar]
- 9.Becker B, Constant MA. The facility of aqueous outflow. A comparison of tonography and perfusion measurements in vivo and in vitro. Arch Ophthalmol 1956;55:305–12. [PubMed] [Google Scholar]
- 10.Mermoud A, Baerveldt G. Aqueous humor dynamics in rats. Graefes Arch Clin Exp Ophthalmol 1996;234:198–203. [DOI] [PubMed] [Google Scholar]
- 11.Vaudaux J, Mermoud A. Aqueous dynamics after deep sclerectomy: in vitro study. Ophthalmic Practice 1998;16:204–9. [Google Scholar]
- 12.Zimmermann TJ, Kooner KS, Ford VJ, et al. Effectiveness of non penetrating trabeculectomy in aphakic patients with glaucoma. Ophthalmic Surg 1984;15:44–50. [PubMed] [Google Scholar]
- 13.Zimmermann TJ, Kooner KS, Ford VJ, et al. Trabeculectomy vs non-penetrating trabeculectomy: a retrospective study of two procedures in phakic patients with glaucoma. Ophthalmic Surg 1984;15:734–40. [PubMed] [Google Scholar]