The occurrence of variant Creutzfeldt-Jakob disease (vCJD) and the probable causal link with bovine spongiform encephalopathy (BSE) in cattle have increased interest in the search for possible environmental sources of sporadic CJD (sCJD). Presumed iatrogenic CJD is rare. Up to the year 2000 there had been 267 cases reported worldwide: three cases secondary to human corneal grafting (one confirmed, one probable, and one possible case), 114 related to human dura mater grafts, 139 related to human growth hormone treatment, four related to human pituitary gonadotrophin therapy, and seven linked to neurosurgical procedures or stereotactic EEG electrodes.1 Because of the marked resistance of the infectious agent of CJD to conventional sterilisation techniques, there is concern about the possibility of transmission of infection via surgical instruments in contact with infected tissue, especially in neurosurgery or ophthalmic surgery.
The presence of infection in the eye in sCJD was first demonstrated following the intracerbral inoculation of pooled sCJD eye tissue in non-human primates.2 Recently the infectious form of prion protein (PrPSc) has been identified in the neural retina, optic nerve, and in retinal pigmented epithelium in variant and sporadic CJD using immunohistochemistry or western blot,3,4 with comparable levels to those found in brain. PrPSc was not detected in other ocular tissues. Although this suggests that there may be a greater risk of contaminating surgical instruments in procedures involving the posterior segment of the eye, infectivity has been demonstrated in animal and human cornea,5 and circumstantial evidence has implicated corneal transplantation as a mechanism of transmission of iatrogenic CJD.6 Experimental infection has been achieved following conjunctival installation of scrapie infectivity in mice7 and by inoculation of an adapted agent into the anterior chamber of the eye in guinea pigs.8
Recently it has been shown that the experimental transmission of metallic suface bound prions is highly efficient.9 Steel wires in contact with the brain of pre-symptomatic mice needed only 5 minutes to acquire an infectious load equivalent to the injection of a 1% homogenate of brain. Infected wires were inserted transiently into the brains of healthy mice and only 30 minutes of exposure was sufficient to result in infection. The same wires remained infective when reintroduced into another set of healthy mice. Although, to our knowledge, there have been no documented cases of CJD secondary to ophthalmic surgery other than corneal transplantation, there is a possibility that ophthalmic surgery might be a risk procedure for the accidental iatrogenic transmission of CJD.
Precautions to minimise the risks of iatrogenic transmission of CJD are vital and the Department of Health has established an incidents panel to provide advice in cases of all forms of human prion diseases in which there is the possibility for cross infection. However, an important question is whether the concerns raised by experimental work translate into an actual risk in the clinical setting. This commentary reviews the data on ophthalmic surgery in sCJD and vCJD from the archives of the UK National CJD Surveillance Unit from 1990 to 2002, including information on both sCJD and vCJD.
We analysed the surgical history of sCJD and vCJD cases with specific reference to ophthalmic surgery. Cases of CJD were identified in the current prospective UK national surveillance project (1990-October 2002) by direct notification or from death certificates,10 and were classified as definite, probable, or possible cases of sCJD or vCJD according to published diagnostic criteria. Only definite or probable cases were included in this analysis. All cases with a history of ophthalmic surgery were identified from the database, which has a specific code for this type of surgery. Information on past ophthalmic surgery was obtained from relatives, general practitioner records, and/or copies of case notes. Case files were examined to identify the type of surgery, date of surgery, and hospital in which the surgery had taken place. In cases in which the surgery was carried out after the onset of clinical symptoms of CJD detailed information on the clinical course was extracted.
The frequency of a history of eye surgery in sCJD and vCJD was compared with data on the frequency of past eye surgery in age and sex matched control groups. During the period of the study the case-control study has evolved. Between 1990 and 1998 a single hospital control was obtained for the sCJD cases and from 1999–2002 a single community based control was identified. From 1996–2002 a single hospital control was obtained for vCJD cases and since 1998 attempts have been made to obtain four community based controls per case of vCJD. Because of the limited numbers of controls and the infrequency of past eye surgery this study reports on unmatched comparisons of the frequency of past eye surgery.
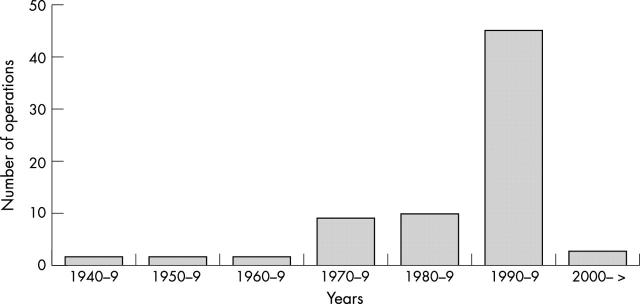
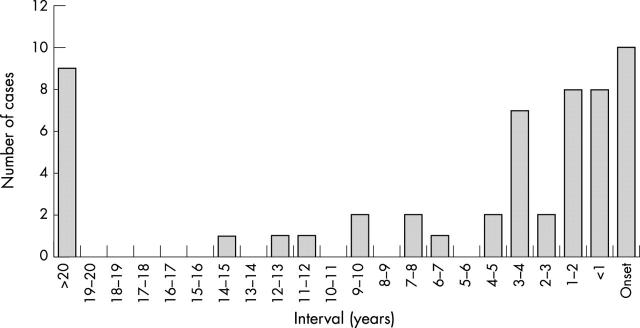
Fifty eight cases of sCJD (11%) out of 510 with information available had a history of intraocular surgery, with an average of 1.34 interventions per patient. The types of operation (n = 78) in the total of 58 cases having undergone any form of ocular surgery are listed in table 1 and the years of operation in figure 1. Ten cases of sCJD underwent eye surgery during the prodromal (within 3 months of onset) or early symptomatic phase of the disease, the majority cataract operations. Four out of these 10 patients had the Heidenhain variant of sCJD, with visual onset and early development of cortical blindness (table 2). Figure 2 shows the time interval between last eye surgery and the onset of symtoms in sCJD patients.
Table 1.
Types of operation on cases of sporadic CJD
| Number of operations on sCJD cases (n = 78) | Number of operations on hospital controls (n = 39) | Number of operations on community controls (n = 20) | |
| Intraocular surgery* | 55 (70%) | 29 (74%) | 17 (85%) |
| Extraocular surgery | 17 (22%) | 6 (16%) | 3 (15%) |
| Laser therapy | 4 (5%) | 2 (5%) | — |
| Information not available | 2 (3%) | 2 (5%) | — |
*Cataract, trauma, and glaucoma.
Figure 1.
Year of eye surgery in sCJD.
Table 2.
sCJD patients with ophthalmic surgery after clinical onset
| Case | Date of surgery | Intervention | Heidenhain variant | Diagnosis |
| 1 | 1992 | Cataract | Definite | |
| 2 | 1992 | Laser therapy | * | Definite |
| 3 | 1992 | Cataract | * | Definite |
| 4 | 1993 | Cataract | Definite | |
| 5 | 1996 | Cataract | Definite | |
| 6 | 1998 | Dacryocystorhinostomy | Definite | |
| 7 | 1998 | Cataract | Definite | |
| 8 | 1999 | Cataract | * | Definite |
| 9 | 1999 | Laser therapy | * | Definite |
| 10 | 2000 | Cataract | Definite |
*Heidenhain variant: cases with isolated cortical visual symptoms at onset.
Figure 2.
Interval between last eye surgery and onset of symptoms in sCJD (n = 54).
The frequency of past eye surgery was compared with the control groups in sCJD (table 3) and vCJD. In the hospital control group for sCJD, 31 (14%) out of 226 had a history of ophthalmic surgery, with an average of 1.26 procedures per patient and in the community control group for sCJD 14 (13%) out of 106, with an average of 1.43 procedures per patient. Eight patients with vCJD (6%) out of 125 with information available had a history of eye surgery and all were squint corrections in childhood, with the exception of one case with a history of open surgery for retinal detachment carried out 15, 17, and 20 months before the development of symptoms (this case predated the establishment of the CJD incidents panel). In the hospital control group for vCJD (15%) 10 out of 67 had a history of ophthalmic surgery and in the community control group for vCJD five (3%) out of 155 had had eye surgery. There were no significant (at the 5% level) differences between the frequencies of past eye surgery in the cases and any of the control groups.
Table 3.
Past ophthalmic surgery in sCJD compared with control groups
| Cases (n = 510) | Hospital controls (n = 226) | Community controls (n = 106) | |
| Patients with eye operations | 58 (11%) | 31 (14%) | 14 (13%) |
| Total number of operations | 78 | 39 | 20 |
| Average per patient | 1.34 | 1.26 | 1.43 |
Details of the year and hospital of each surgical procedure were listed and in the great majority there was no temporal or geographic link between operations. A group of six cases of sCJD had been operated on in one hospital and in two pairs of cases the procedures had been carried out in the same year. Inquiry about the specific dates of these procedures, however, indicated that the operations had been carried out months apart.
The aim of this article is to document the frequency of past eye surgery in CJD and to determine whether there is evidence of transmission of CJD through contaminated ophthalmic instruments. About 10% of cases of sCJD have a history of eye surgery, of which about 70% involved open surgery on the anterior chamber of the eye. In the great majority of cases the surgical instruments were reused on subsequent patients, usually because CJD developed years after the original procedure. Despite this, the evidence in this paper does not suggest that there is onward iatrogenic transmission of sCJD through eye surgery, a finding consistent with some previous studies.11,12
An important question is whether cases of CJD caused by this type of transmission would be identified by this study. The clinicopathological features and incubation period of iatrogenic CJD vary according to the route of transmission with, for example, a cerebellar syndrome and only limited cognitive impairment in human growth hormone related CJD. In CJD caused by corneal transplantation the clinical and pathological phenotype is similar to sCJD and the incubation periods in the three cases reported to date were 15 months, 18 months, and 30 years.6,13 The surveillance system for CJD in the United Kingdom is efficient at identifying cases as judged by annual incidence rates of sCJD of around one case per million, and it is likely that cases with a typical phenotype would be identified. The period of observation of CJD from 1990 to 2002, with additional information from 1980–9, suggests that there is the potential to identify case to case transmission through past eye surgery, with the assumption that the incubation period of such cases would be months or years rather than decades. It is, however, important to emphasise that a significant proportion of the eye operations were carried out relatively recently and, if the incubation period were extended, onward transmission may not have been identified by this study.
Evidence of transmission of sCJD through contaminated neurosurgical instruments rests on the close temporal relation between operations carried out on CJD cases and unaffected individuals who subsequently developed CJD.14,15 An analysis of the dates and sites of eye operations in cases of sCJD in this study showed no such relation. The elegant experiments by Weismann et al9 raise the possibility that surgical instruments contaminated during neurosurgery might pose a significant risk of iatrogenic transmission. In this context it is of interest that a number of cases of sporadic (and iatrogenic) CJD have undergone brain surgery (12/400 cases in the European study), but there is no evidence from case-control studies that previous neurosurgery increases the risk of developing sporadic CJD. This is despite the fact that potentially contaminated neurosurgical instruments have inadvertently been reused on other patients on a number of occasions.
The 10 cases of sCJD undergoing eye surgery in the early clinical stages of the illness may represent the greatest risk of onward transmission of infection, because there are probably higher levels of infection in the eye late in the incubation period as a result of centrifugal spread of infection along the optic nerve.4 The diagnosis of sCJD can be very difficult in the early stages of the clinical illness and this is particularly true of cases with isolated cortical visual symptoms (the Heidenhain variant of sCJD), which affected four of these 10 cases. The presence of confusion, memory impairment, or other neurological signs may raise the suspicion of a neurodegenerative disorder.
The data on vCJD are limited and the period of observation is shorter than in sCJD. Although there is currently no evidence of transmission of vCJD through contaminated ophthalmic instruments, this possibility cannot be excluded, not least because the incubation period in vCJD is unknown. Furthermore, in vCJD the presence of prion protein (PrP) immunostaining in systemic lymphoreticular tissues suggests that the risk from contaminated surgical instruments may be greater than in sCJD, in which these tissues do not stain for PrP, using comparable methods.
The data from our case-control study show no significant risk related to a history of eye surgery in sCJD in comparison with two control groups. The methodology of the case-control study in our analysis is not ideal. There is a deficit in the numbers of controls in comparison with the numbers of cases and an unmatched analysis was undertaken. The results of our study are, however, consistent with the results of a previous case-control study in Europe,12 but contrast with the results of an Australian study in which previous cataract/eye surgery was associated with a more than sixfold increase in the risk of sCJD.16 The studies used different types of control group, the European study hospital controls and the Australian study community controls. However, in a reanalysis of the European data using a community control group, eye surgery was again found not to increase the risk of sporadic CJD.11 The disparity in outcome in the two studies relates primarily to the frequency of previous eye surgery in the control groups (European studies 34/406, 8%, 37/325, 11%: Australian 24/784, 3%) rather than the cases (European studies 33/401, 8%, 37/328, 11%: Australian 24/241, 10%). Although this type of study cannot exclude the possibility of rare instances of iatrogenic transmission of CJD through eye surgery, the balance of evidence does not support the hypothesis that exposure to potentially contaminated ophthalmic instruments has, hitherto, been associated with the risk of developing CJD.
Although the evidence in this paper does not suggest that contaminated ophthalmic instruments represent a risk of onward transmission of sporadic CJD, this conclusion should be treated with caution. The eye contains significant levels of infection in sCJD and vCJD, and even limited exposures can result in the iatrogenic transmission of CJD.17
This paper confirms that ophthalmologists may be involved in treating patients in the early stages of CJD and it is essential to follow current guidelines in relation to surgical, including ophthalmic and neurosurgical, instruments used in suspect cases of CJD of all types.18 Such instruments should be destroyed or quarantined until a definitive diagnosis is available and the development of single use devices and instruments in eye surgery has been advocated,19 provided these do not prejudice clinical outcome. All cases of suspect CJD should be reported to the local consultant in communicable disease control in order that appropriate measures to protect public health are instituted, including a review of previous surgery. Advice on specific cases which raise concern is available from the CJD incidents panel (www.doh.gov.uk/cjd/incidentspanel.htm), which has published a consultation document (www.doh.gov.uk/cjd/consultation).
Acknowledgments
We would like to thank Dr JF Geddes and Miss Gillian Adams, FRCS, for their help in confirming some of the clinical details. PS-J was supported by the postMIR grant Wenceslao Lopez Albo from the IFIMAV Institute of the Fundación Pública Marqués de Valdecilla. The National CJD Surveillance Unit is funded by the Department of Health and the Scottish Executive Health Department.
REFERENCES
- 1.Brown P, Preece M, Brandel JP, et al. Creutzfeldt-Jakob disease at the millennium. Neurology 2000;55:1075–81. [DOI] [PubMed] [Google Scholar]
- 2.Brown P, Gibbs CJ, Rodgers-Johnson P, et al. Human spongiform encephalopathy: The National Institutes of Health Series of 300 cases of experimentally transmitted disease. Ann Neurol 1994;35:513–29. [DOI] [PubMed] [Google Scholar]
- 3.Wadsworth JD, Joiner S, Hill AF, et al. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 2001;358:171–80. [DOI] [PubMed] [Google Scholar]
- 4.Head MW, Northcott V, Rennison K, et al. Prion protein accumulation in eyes of patients with sporadic and variant Creutzfeldt-Jakob disease. Invest Ophthalmol Vis Sci 2002;44:342–6. [DOI] [PubMed] [Google Scholar]
- 5.Lueck CJ, McIlwaine GG, Zeidler M. Creutzfeldt-Jakob disease and the eye. I Background and patient management. Eye 2000;14:263–90. [DOI] [PubMed] [Google Scholar]
- 6.Hogan RN, Brown P, Heck E, et al. Risk of prion disease transmission from ocular donor tissue transplantation. Cornea 1999;18:2–11. [PubMed] [Google Scholar]
- 7.Scott JR, Foster JD, Fraser H. Conjunctival instillation of scrapie in mice can produce disease. Vet Microbiol 1993;34:305–9. [DOI] [PubMed] [Google Scholar]
- 8.Manuelidis EE, Angelo JN, Gorgacz EJ, et al. Experimental Creutzfeldt-Jakob disease transmitted via the eye with infected cornea. N Eng J Med 1977;296:1334–6. [DOI] [PubMed] [Google Scholar]
- 9.Weissmann C, Enari M, Klohn P-C, et al. Transmission of prions. J Infect Dis 2002;186:S157–65. [DOI] [PubMed] [Google Scholar]
- 10.Cousens SN, Zeidler M, Esmonde TF, et al. Sporadic Creutzfeldt-Jakob disease in the United Kingdom: analysis of epidemiological surveillance data for 1970–76. BMJ 1997;315:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward HJT, Everington D, Croes EA, et al. Sporadic Creutzfeldt-Jakob disease and surgery: a case control study using community controls. Neurology 2002;59:543–8. [DOI] [PubMed] [Google Scholar]
- 12.Van Duijn CM, Delasnerie-Lauprêtre N, Masullo C, et al. Case control study of risk factors of Creutzfeldt-Jakob disease in Europe during 1993–95. Lancet 1998;351:1081–5. [DOI] [PubMed] [Google Scholar]
- 13.Heckmann JG, Lang CJG, Petruch F, et al. Transmission of Creutzfeldt-Jakob disease via corneal transplant. J Neurol Neurosurg Psychiatry 1997;63:388–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Will RG, Matthews WB. Evidence for case-to-case-transmission of Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry 1982;45:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foncin JF, Gaches J, Cathala F, et al. Transmission iatrogene interhumaine possible de maladie de Creutzfeldt-Jakob avec atteinte des grains de cervelet. Rev Neurol (Paris) 1980;136:280. [Google Scholar]
- 16.Collins S, Law MG, Fletcher A, et al. Surgical treatment and risk of sporadic Creutzfeldt-Jakob disease: a case-control study. Lancet 1999;353:693–7. [DOI] [PubMed] [Google Scholar]
- 17.Croes EA, Roks CMAA, Jansen GH, et al. Creutzfeldt-Jakob disease 38 years after diagnostic use of human growth hormone. J Neurol Neurosurg Psychiatry 2002;72:792–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Health. Transmissible spongiform encephalopathy agents: safe working and the prevention of infection: publication of revised ACDP/SEAC guidance. London: DoH, June2003. (www.doh.gov.uk/cjd/tseguidance).
- 19.Tullo A. Creutzfeldt-Jakob disease and eye surgery—new disease, old disease. J Cataract Refract Surg 2003;29:629–31. [DOI] [PubMed] [Google Scholar]