Abstract
Background/aim: Structural changes in the lamina cribrosa have been implicated in the pathogenesis of glaucomatous optic atrophy. The aim of this study was to determine a measure the surface variability of the cup floor in normal subjects and patients with glaucoma.
Methods: A sample of age matched normal subjects (NN), patients with low tension glaucoma (LTG), and primary open angle glaucoma (POAG) were included in the study. The glaucoma groups were matched for the severity of the visual field loss. Mean 10 degree topographic images of normal and glaucomatous eyes from the Heidelberg retina tomograph were imported into ERDAS image processing software where topographic analysis of the cup floor could be assessed. Each image was processed using customised spatial filters that calculated the surface depth variation in localised neighbourhood areas across each image. The local change in depth across the cup floor surface was determined and compared between the three clinical groups.
Results: The depth variation in the cup floor was largest in normal subjects followed by LTG and POAG. Highly statistically significant differences in surface depth variability of the cup floor existed between normal and LTG (p = 0.005), between normal and POAG (p<0.0001), and between LTG and POAG groups (p<0.0001). The variability and skewness of depth difference across the optic cup floor were also significantly different between the three clinical groups.
Conclusion: A new parameter quantifying depth variations in the cup floor significantly discriminated between groups of normal and glaucoma patients. This new parameter may contribute to a better understanding of the pathogenesis of the glaucomatous optic nerve damage in different types of glaucoma.
Keywords: lamina cribrosa, glaucoma
The lamina cribrosa is a sieve-like membrane in the external ocular layers, through which the retinal ganglion cell axons pass before condensing to form the optic nerve. The lamina cribrosa is partially hidden behind the converging retinal nerve fibres and generally only a small portion is visible at the base of the optic cup in normal eyes.
Structural changes in the lamina cribrosa have been implicated in the pathogenesis of glaucomatous optic atrophy. Thus, more precise measurements of the membrane’s structure may provide valuable insights into the glaucomatous process.1–4
Limited viewing of the lamina cribrosa has made it difficult to study this structure and most of the information that is available is derived from postmortem studies or from direct observation.5–8 The advent of scanning laser technology has resulted in the development of techniques for imaging the lamina cribrosa in vivo.4,9–12
In previous research by Morgan-Davies et al13 a localised measure of the surface variability of the visible lamina cribrosa revealed significant differences between normal, ocular hypertensive, and glaucomatous groups and appeared to reflect glaucoma related changes. In this study, a new technique has been designed to assess the overall surface variability of the cup floor. This variability is a measure of the surface depth change in the base of the optic cup. The measurements are derived using customised spatial filters to transform images obtained from the Heidelberg retina tomograph (Heidelberg, Germany).
SUBJECTS AND METHODS
Three age matched groups of patients consisting of normal, primary open angle glaucoma (POAG), and low tension glaucoma (LTG) were randomly selected. Patients were recruited from an academic department of ophthalmology. Normal subjects were recruited from members of the hospital staff. One eye per subject was randomly chosen. Informed consent was obtained in all cases.
The three subject groups in the study were defined as follows: (1) normal (N), best corrected visual acuity of 6/12 or better, no visual field defect on Humphrey visual field analysis (program 24–2), intraocular pressure (IOP) less than 22 mm Hg, and normal appearance of the optic nerve head by slit lamp biomicroscopy; (2) primary open angle glaucoma (POAG), best corrected visual acuity of 6/12 or better, reproducible glaucomatous visual field defects on Humphrey visual field analysis (on pattern deviation plot, a cluster of three or more non-peripheral points in an expected location depressed below the 5% level, at least one of which is depressed below the 1% level), IOP greater than 21 mm Hg on two separate occasions, and open anterior chamber angle; (3) low tension glaucoma (LTG): as for POAG with the exception of IOP less than 21 mm Hg on diurnal phasing. Patient groups were age matched to eliminate any possible effect that ageing may have had on the lamina cribrosa. The POAG group and LTG groups were matched for the extent of visual field loss and disc area. Patients with poor quality of Heidelberg retina tomograph (HRT) images (see below for details) and refractive errors exceeding plus or minus 3 dioptres of spherical equivalent were excluded. Eight normal subjects, seven patients with POAG, and 10 with LTG were studied.
In order to focus on independent measures within the lamina, and avoid the influence of disc size in the lamina cribrosa appearance (for example, small discs with prominent vessels), a predetermined range of disc areas was selected for inclusion limiting the range from 2.2 mm2 to 2.6 mm2. This range was defined by obtaining the mean and standard deviation of the disc size for the group of normal subjects and patients from the archive (2.4 mm2) and allowing discs within the range of plus or minus 2 SD of the mean to be included. A total of 25 eyes from 25 subjects were analysed: eight normal subjects from an initial sample of 12, seven patients with POAG from an initial sample of 11, and 10 LTG from an initial sample of 15 (table 1).
Table 1.
Demographic, disc, and visual field variables
| Group | No | Age (years) | Sex | Disc area (mm2) | Deviation (dB) |
| Mean (SD) | M : F | Mean (SD) | Mean (SD) | ||
| Normal | 8 | 66.75 (4.65) | 2:6 | 2.11 (0.12) | 0.001 (0.56) |
| POAG | 7 | 68.14 (6.52) | 2:5 | 2.18 (0.22) | −11.73 (9.59) |
| LTG | 10 | 66.8 (9.95) | 3:7 | 2.21 (0.18) | −11.22 (7.55) |
| p Value | 0.92 | 0.97 | 0.52 | <0.003 |
The p value refers to the ANOVA significance of the difference across all three groups.
Image capture
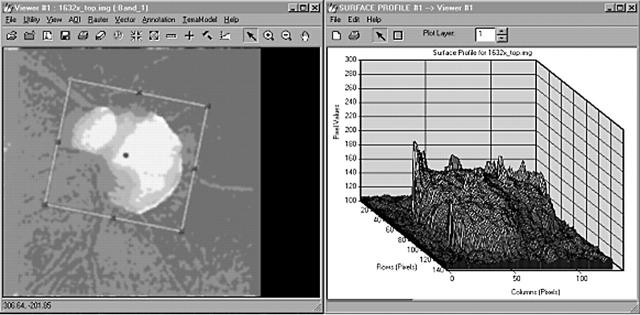
Images of the optic nerve head surface were produced using the Heidelberg retina tomograph (HRT, software version 1.11). The HRT archive of normals comprised 50 subjects with a mean disc area of 2.0 mm2. For each subject, three 10° images centred on the optic disc were taken and a mean topography image was created. The HRT images were exported as generic TIFF files into ERDAS image processing software (ERDAS Imagine 8.4 for WinNT 4.0). The main functions of ERDAS were to manipulate, enhance, and resolve image data within the spatial domain and output to statistical or further spatial analysis software. The depth values for each image were defined using the HRT software and were not modified by the EDRAS program. Although the HRT assigns the depth values as a colour coded topography image with respect to the surface of the papillomacular nerve fibre bundle layer, the depth values at the floor of the optic cup were specified in the EDRAS program in terms of the relative luminance values for each pixel. The result was that the EDRAS program preserved topographic data in the same way as in the HRT image. After importation, 3D surface images (figs 1 and 2) were created and scrutinised by a single observer (JMD) masked for the diagnosis. Image quality was determined by inspecting the clarity of blood vessels at the optic nerve rim and focusing the instrument in the normal way. Only images with a topographic mean pixel height standard deviation <30 μm were included in the study.
Figure 1.
Mean image imported from the Heidelberg retina tomograph (HRT), (left), and the surface profiling tool (ERDAS) (right). The ordinate in the three dimensional plot of the digitised image is the luminance associated with each pixel and, therefore, represents different depths in the lamina cribrosa surface across the floor of the optic cup.
Figure 2.
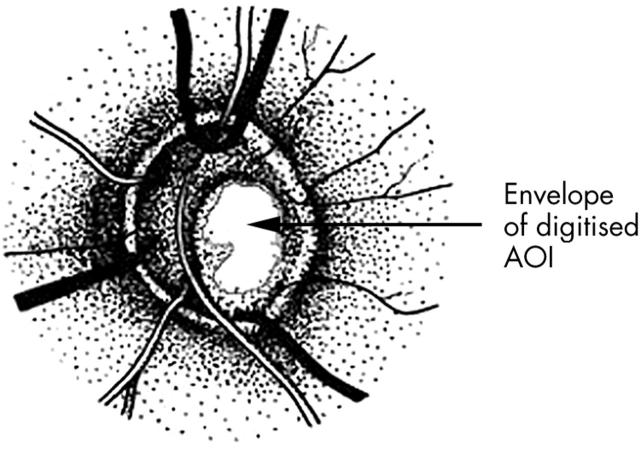
Example of an optic disc showing the delineated area of interest (AOI) of the cup floor selected to be digitised. The AOI specifically excluded vessels in the optic cup.
Area of interest selection
The cup floor (bounded by the cup rim) was digitised to create an AOI and the data within the polygon extracted as a subset of the original (fig 3). While the initial matching procedure meant that there was only a small range of cup size between the three clinical groups (2.2 mm2 to 2.6 mm2), there were inevitable differences in the AOI which was defined to exclude disc blood vessels. The mean/range of the AOI across all images expressed as the number of pixels for the three groups were respectively as follows: normal 832/1368; POAG 1690/2074, and LTG 1636/2319.
Figure 3.
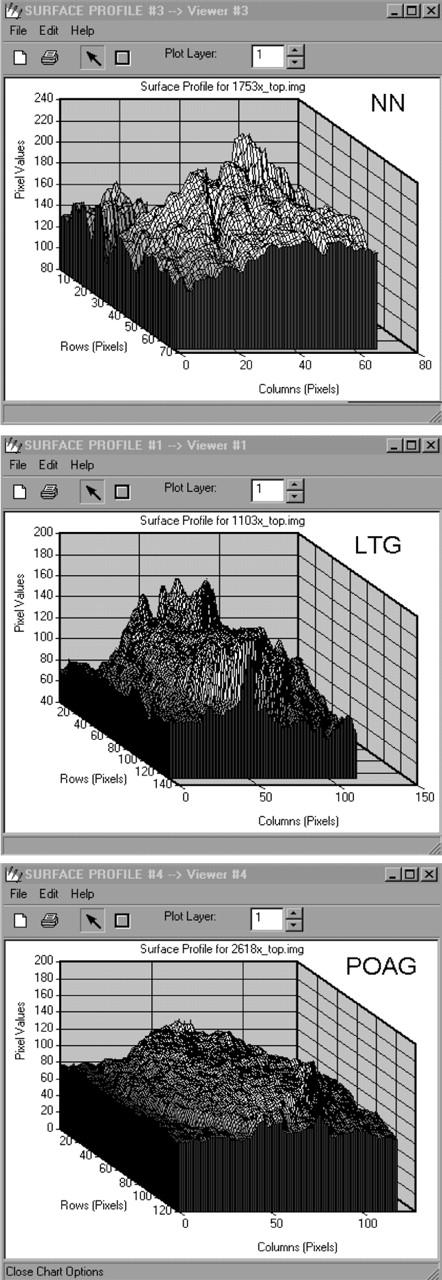
Surface plots (ERDAS) showing surface variations of the lamina cribrosa in a normal subject (NN, top), low tension glaucoma patient (LTG, middle), and primary open angle glaucoma patient (POAG, bottom). (Note the different range in the ordinate axes of the three figures.)
Surface variability analysis
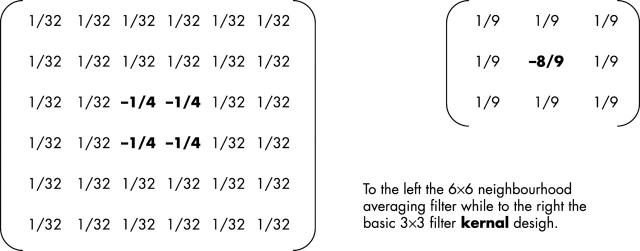
Because optic cups had highly irregular surfaces, a processing filter kernal (fig 4) termed a “neighbourhood averaging” filter was applied. In order to measure surface variability, a 6×6 matrix or window was placed on the floor of the optic cup. Each of the 36 cells making up the window corresponded to a single pixel or data point on the optic cup image. For each location of the matrix the average luminance value (that is, depth) of the neighbouring cells as a reference for the centre cell was calculated. The value calculated for the centre cell was the difference between its actual depth value and the average depth of its neighbours. If there were no depth difference between a cell and its neighbours then that part of the surface would be relatively flat. Departure from zero depth difference indicates surface variability. The matrix was then moved one cell to the right and the procedure repeated. The procedure produced, therefore, an array of difference values of the surface at the floor of the optic cup. These difference values reflecting surface variability were used as the dependent variable in the analysis.
Figure 4.
The neighbourhood averaging filter. A 6×6 matrix (left) was placed on the floor of the optic cup. Each of the 36 cells corresponded to a single pixel or data point on the optic cup image. For each location of the matrix the average depth of the neighbouring cells was calculated as a reference for the centre cell. The value calculated for the centre cell was the difference between its actual depth value and the average depth of its neighbours. If there was no depth difference between a cell and its neighbors then that part of the surface would be relatively flat. Departure from zero depth difference indicates surface variability.
The neighbourhood averaging filter used in the study was based upon a standard 3×3 smoothing filter and used an extrapolation function that enhanced strong signals by spreading across four rather than the single central pixel. The neighbourhood function then replaced these central pixels in the image with the average of the differences between the pixel’s value and the values of its neighbouring pixels. Thus, given that each pixel’s luminance value is a measure of its depth (because of the laser scanning technology employed by the HRT), the result of applying the neighbourhood filter was to produce an image in which each new pixel value represented the local “depth change” found at that point in the original image.
The AOI subset image was scanned by the neighbourhood averaging filter producing a new output image, a ×3 multiplication function was then applied to the output data to scale the data range. Finally, the artefactual boundary around the data caused by the effect of the processing filter was removed by re-digitising. The basic luminance values for each pixel were exported into a standard statistical package (SPSS) and, because the distributions were highly skewed, non-parametric statistical analyses were used to compare differences between the three groups.
A 3% random sample of the data was used to carry out the statistical analysis. This was chosen as a consequence of the sequential scanning strategy of the filter used. The filter calculated the difference between centre pixels and the values of its neighbours written to the output then moved one step to the right to repeat, thus overlapping itself as it scans. Since the filter was a 6×6 matrix, a 3% sample only analysed non-overlapping data, or approximately every 36th pixel. The small size of the random sample did not present a problem, as there were 36 000 measures across the patient groups and around 1400 measures per patient.
The distribution of pixel luminance values were highly skewed so a Kruskall-Wallis non-parametric analysis of variance was calculated to test the overall statistical significance of the differences among the three groups. Paired comparisons between groups were then carried out using the Mann-Whitney U test. Probabilities of 0.05 or below were considered statistically significant.
RESULTS
Demographics, disc area, and mean defect are summarised in table 1. There was no statistically significant difference among the three matched groups in terms of sex (p = 0.97), age (p = 0.92), and optic disc size (p = 0.52). The difference among the three groups in terms of mean deviation values (MD) was highly significant (p<0.003, table 1), although the differences in severity of visual field loss (that is, MD) between the matched POAG and LTG groups were not statistically significant (p = 0.775).
The depth variation in the lamina cribrosa was largest in normal subjects followed by LTG and POAG. The resulting χ2 for a total of 1000 measures across the three patient groups was 26.7 with two degrees of freedom. This was highly statistically significant (p<0.001) showing clear differences in depth values between the three groups.
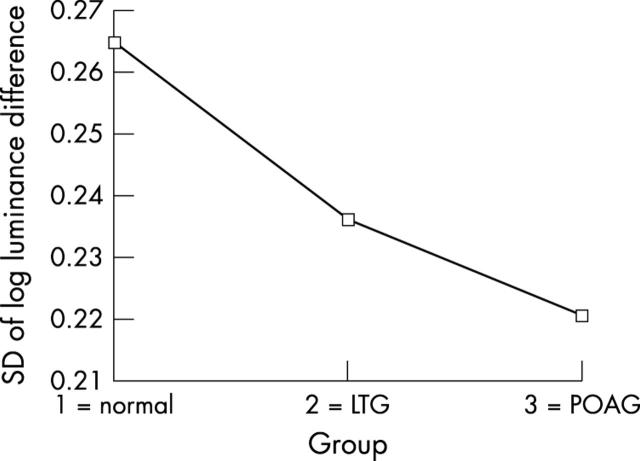
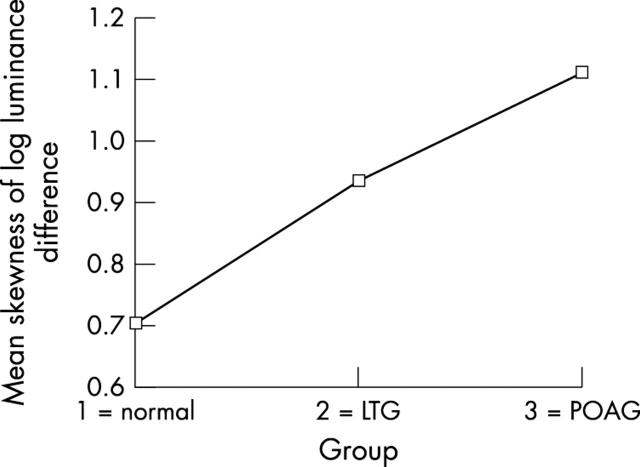
Table 2 shows the differences between groups in the surface variability values (Mann-Whitney U test). The results shown in all paired difference tests confirm the hypothesis that patients with POAG, LTG, and normal subjects differ considerably on the measure of surface variability across the floor of the optic cup. Mean variability was greatest in normals, least in POAG with an intermediate mean value for LTG. This effect was examined further using the standard deviation of four independent random samples of one quarter fractional replicated of the pixel array for each image as an index of topographic variability. Using a mixed factorial design with repeated measures, an analysis of variance showed a highly significant main effect of topographic variability across the three groups (see table 3 and fig 5). Further inspection of this variability shows that it is not randomly distributed across the cup floor. In LTG and POAG there is an accumulation of clustered greater depth values in the superior and inferior regions of the cup floor (fig 6). One index of this clustering effect is a measure of skewness of the distribution of pixel luminance values across the cup floor. Four indices of skewness were obtained for each image from four random samples of one quarter fractional replicates of the pixel array. Using a similar mixed factorial design with repeated measures as above, an analysis of variance showed highly significant differences in the distribution of skewness indices between the three groups (see table 4). Figure 7 shows that there is a progressive increase in these skewness indices of pixel luminance between the three groups from normal, through LTG to POAG, indicating significantly greater clustering of depth values in LTG and POAG compared with normals.
Table 2.
Paired comparisons (Mann-Whitney U test) between the three clinical groups groups of the surface variability values
| Normal | LTG | POAG | |
| Normal | – | Z = 2.831 | Z = 5.41 |
| p = 0.005 | p<0.0001 | ||
| LTG | – | Z = 4.17 | |
| p = <0.0001 | |||
| POAG | – |
Table 3.
ANOVA between groups and images. Measure: SDLOGHT
| Source | df | Mean square | F | Significance |
| GROUP | 2 | .014 | 3094.770 | 0.000 |
| Error(GROUP) | 6 | 4.569E-06 | ||
| IMAGE | 6 | .009 | 2462.609 | 0.000 |
| Error(IMAGE) | 18 | 3.762E-06 | ||
| GROUP * IMAGE | 12 | .008 | 2670.564 | 0.000 |
| Error(GROUP*IMAGE) | 36 | 3.040E-06 |
ANOVA for a mixed design with repeated measures using four independent random one quarter fractional estimates per image of the standard deviation of pixel luminance across the cup floor as the dependent variable. (The group variable represents the three clinical groups of normal, low tension glaucoma and primary open angle glaucoma).
Figure 5.
Optic cup floor variability across the three clinical groups represented as the standard deviation of pixel log luminance difference. (LTG = low tension glaucoma, POAG = primary open angle glaucoma).
Figure 6.
The spatial distribution of pixel luminance difference across the optic cup floor in an eye with primary open angle glaucoma. This shows a clustering of values associated with small areas of greater depth in the superior and inferior regions of the cup floor.
Table 4.
ANOVA between groups and images. Measure: SKEWNESS
| Source | df | Mean square | F | Significance |
| GROUP | 2 | 1.194 | 674.005 | 0.000 |
| Error(GROUP) | 6 | 0.002 | ||
| IMAGE | 6 | 0.538 | 53.857 | 0.000 |
| Error(IMAGE) | 18 | 0.010 | ||
| GROUP * IMAGE | 12 | 0.733 | 66.844 | 0.000 |
| Error(GROUP*IMAGE) | 36 | 0.011 |
ANOVA for a mixed design with repeated measures using four independent random one quarter fractional estimates per image of the skewness of the pixel luminance distribution across the cup floor. (The group variable represents the three clinical groups of normal, low tension glaucoma, and primary open angle glaucoma.)
Figure 7.
Differences between the three clinical groups in the randomness of small areas of greater depth across the cup floor expressed in terms of the skewness index of pixel log luminance difference distribution. (LTG = low tension glaucoma, POAG = primary open angle glaucoma.)
DISCUSSION
The new parameter of surface variability of the optic disc cup showed significant differences between the glaucomatous and non-glaucomatous groups. The new measure is an objective assessment that can be carried out to assess the variation in surfaces within optic discs. This follows similar findings from a previous study13 evaluating localised depth variability of the lamina cribrosa.
These findings raise a number of questions. It is possible that as the glaucomatous damage to the optic nerve tissues progresses the surface variability of the lamina cribrosa is reduced. Another possible factor would be that the elevation of intraocular pressure could contribute to the reduction of height variability. However, an inherent anatomical property of optic discs that would predispose to glaucomatous damage cannot be ruled out. Thus, normal subjects having greater depth variability values and POAG patients having smaller depth variability values might represent opposite ends of the spectrum of anatomical variability and not acquired changes. The relation between cup shape and topography of the floor cup was explored in a previous paper13 which showed that as the cup shape measure increased, the topography irregularity decreased with a correlation of −0.42 (p<0.002).
It is known that the pores in the superior and inferior quadrants of the lamina cribrosa are larger in normal and POAG eyes than those in the horizontally placed nasal and temporal quadrants.14 The visual output of the parameter (fig 4) also suggests that surface variability varies greatly in different parts of the disc and is not randomly distributed. This is confirmed by parametric analysis as shown in figures 5 and 6 and tables 3 and 4. It is possible that the measure could be used to compare variability for different segments within the optic disc. In particular, this indicates the possible ability of such a surface measure to analyse known local morphological disc changes such as focal notch and acquired pit of the optic nerve.
Differences in optic nerve appearance between LTG and POAG have been reported previously.15–19 The ability of the new parameter to discriminate between the different disease groups (particularly between POAG and LTG) may prove useful in contributing to a better understanding of the pathogenesis of different types of glaucoma. If the lamina cribrosa and the base of the optic cup were not characteristically the same in LTG as in POAG then it would be possible to speculate that different pathophysiological mechanisms may be involved in these conditions.
Although the results presented in this study were obtained from a relatively small number of patients, the database from which the random sampling was taken was very large. It is surprising however that given the high surface variability apparent on visual inspection (fig 4), highly statistical significant differences were noted. Further investigation (with a larger patient data archive) into more precise estimates of this surface variation and in its anatomical basis would be warranted.
In summary, the new measure of surface variability at the floor of the optic cup based upon neighbourhood averaging filters has been shown to give objective measures of variability that may represent a measurement of physiological change in the lamina cribrosa. This parameter significantly discriminates between groups of normal and glaucoma patients and suggests a rank order of decreasing variability from normal, through LTG to POAG.
Abbreviations
AOI, area of interest
HRT, Heidelberg retina tomograph
IOP, intraocular pressure
LTG, low tension glaucoma
POAG, primary open angle glaucoma
The authors wish to thank the International Glaucoma Association for funding this project.
REFERENCES
- 1.Minkler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci 1977;16:426–41. [PubMed] [Google Scholar]
- 2.Quigley HA, Anderson DR. The dynamics and location of axonal transport blockage by acute intraocular pressure elevation in primate optic nerve. Invest Ophthalmol Vis Sci 1976;15:606–16. [PubMed] [Google Scholar]
- 3.Quigley HA, Hohman R, Addicks EM, et al. Morphologic changes in the lamina cribrosa correlated with neural loss in open angle glaucoma. Am J Ophthalmol 1983;95:673–91. [DOI] [PubMed] [Google Scholar]
- 4.Fitzke FW, Masters BR. Three dimensional visualisation of confocal sections of human fundus and optic nerve. Curr Eye Res 1993;12:1015–18. [DOI] [PubMed] [Google Scholar]
- 5.Miller KM, Quigley HA. The clinical appearance of the lamina cribrosa as a function of the extent of glaucomatous optic nerve damage. Ophthalmology 1988;95:135–8. [DOI] [PubMed] [Google Scholar]
- 6.Radius RL. Regional specificity in anatomy at the lamina cribrosa. Arch Ophthalmol 1981;99:478–80. [DOI] [PubMed] [Google Scholar]
- 7.Radius RL, Gonzales M. Anatomy of the lamina cribrosa in human eyes. Arch Ophthalmol 1981;99:2159–62. [DOI] [PubMed] [Google Scholar]
- 8.Radius RL, Bade B. Axonal transport interruption and anatomy in the lamina cribrosa. Arch Ophthalmol 1982;100:1661–4. [DOI] [PubMed] [Google Scholar]
- 9.Bhandari A, Fontana L, Fitzke FW, et al. Quantitive analysis of the lamina cribrosa in vivo using a scanning laser ophthalmoscope. Curr Eye Res 1997;16:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Fontana L, Bhandari A, Fitzke FW, et al. In vivo morphometry of the lamina cribrosa and its relation to visual field loss in glaucoma. Curr Eye Res 1998;17:363–9. [DOI] [PubMed] [Google Scholar]
- 11.Maeda H, Nakamura M, Yamamoto M. Morphometric features of laminar pores in lamina cribrosa observed by scanning laser ophthalmoscopy. Jpn J Ophthalmol 1999;43:415–21. [DOI] [PubMed] [Google Scholar]
- 12.Miglior S, Rossetti L, Lonati C, et al. Scanning laser ophthalmoscopy of the optic disc at the level of the lamina cribrosa. Curr Eye Res 1998;17:453–61. [DOI] [PubMed] [Google Scholar]
- 13.Morgan-Davies J, King A, Aspinall A, et al. Measurement of a novel optic oisc parameter, “spikiness” in glaucoma. Graefes Arch Clin Exp Ophthalmol 2000;238:669–676. [DOI] [PubMed] [Google Scholar]
- 14.Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol 1981;99:137–43. [DOI] [PubMed] [Google Scholar]
- 15.Caprioli J, Spaeth GL. Comparison of the optic nerve head in high- and low-tension glaucoma. Arch Ophthalmol 1985;103:1145–9. [DOI] [PubMed] [Google Scholar]
- 16.Javitt JC, Spaeth GL, Katz LJ, et al. Acquired pits of the optic nerve. Increased prevalence in patients with low-tension glaucoma. Ophthalmology 1990;97:1038–44. [DOI] [PubMed] [Google Scholar]
- 17.Miller KM, Quigley HA. Comparison of optic disc features in low-tension and typical open-angle glaucoma. Ophthalmic Surg 1987;18:882–9. [PubMed] [Google Scholar]
- 18.Yamagami J, Araie M, Shirato S. A comparative study of optic nerve head in low- and high-tension glaucomas. Graefes Arch Clin Exp Ophthalmol 1992;230:446–50. [DOI] [PubMed] [Google Scholar]
- 19.Eid TE, Spaeth GL, Moster MR, et al. Quantitative differences between the optic nerve head and peripapillary retina in low-tension and high-tension primary open-angle glaucoma. Am J Ophthalmol 1997;124:805–13. [DOI] [PubMed] [Google Scholar]