Figure 1.
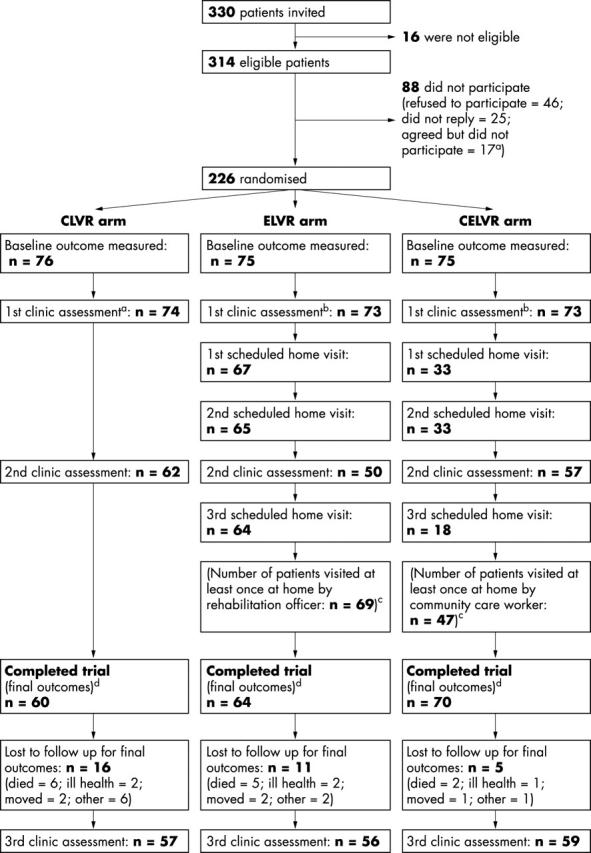
Study design. aNon-refusals who were not recruited: two patients died soon after consenting; 10 consented too late (that is, had consented after attending their first clinic assessment); four consented but lived too far away from the hospital; researcher failed to visit one patient because of researcher’s ill health. bFirst clinic assessments: two patients in CLVR arm died before their appointments; two patients in ELVR arm did not attend, one because of ill health and one for unknown reasons; one patient in CELVR arm died before appointment and one did not attend because of ill health. cHome visits: these are shown in the diagram as they were scheduled. However, because some visits had to be rearranged, the sequence of the home visits varied with respect to second clinical assessment varied for some patients. dDuration of follow up: mean durations of follow up (number of days between the first home visit to measure baseline outcomes and the last home visit to measure outcomes after 12 months’ follow up) were 361, 364, and 362 in CLVR, ELVR, and CELVR arms, respectively.
