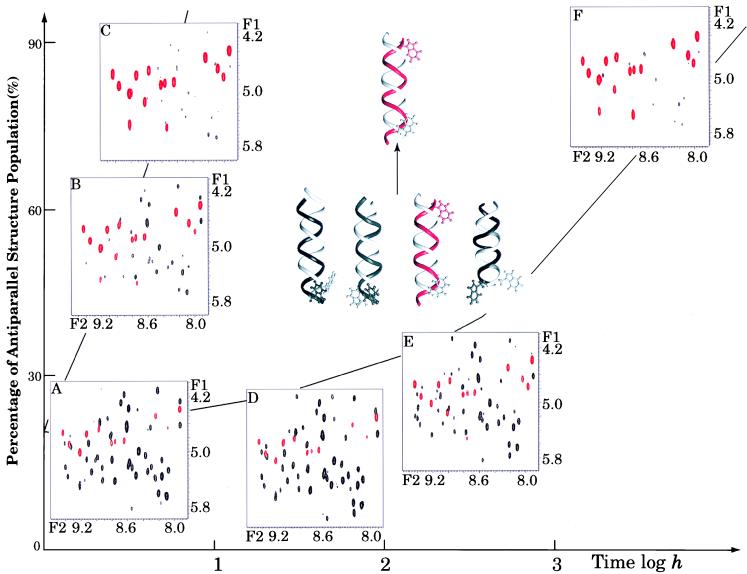
Figure 1.
The GCOSY fingerprint regions for gA (12 mM) solutions during structural rearrangement in the presence (A–C) and “absence” (D–F) of water. The red cross peaks in the spectra represent the backbone NH-CαH cross peaks of an antiparallel left-handed helical dimer (species 3), whereas the black cross peaks are from a mixture of parallel conformers. The horizontal axis is the actual time scale within which the NMR spectra were acquired and the vertical axis corresponds to the increase of the species 3 population over time. The initial and final peptide structures are shown as dimers with red/white double strands for the species 3 structure and black/white strands for the parallel structures. Trp15 at the peptide C terminus is displayed to emphasize the relative orientation of the two peptide strands.