Abstract
Background and aims: The role of Helicobacter pylori and atrophic gastritis in the pathogenesis of gastric lesions in familial adenomatous polyposis (FAP) has not been clarified.
Patients: Thirty one patients with FAP.
Methods: The presence of fundic gland polyposis (FGP) and gastric adenoma (GA) was determined by upper endoscopy with biopsies. The degree of gastric mucosal atrophy and H pylori status were determined by serological and histological findings. Germline mutation in the adenomatous polyposis coli (APC) gene was determined by polymerase chain reaction based single strand conformation polymorphism and direct sequencing.
Results: Gastric lesions were detected in 23 patients (74%). FGP and GA were found in 52% and 39%, respectively. APC gene mutation was identified in 22 of 30 patients. Patients with FGP were less frequently infected with H pylori than those without FGP (13% v 67%). The former patients had a lower degree of atrophy than the latter. Patients with GA tended to be more frequently infected with H pylori and they had higher degrees of atrophy than those without GA. When subjects were subdivided by gastric lesions (FGP alone, FGP+GA, GA alone, and negative groups), the GA alone group had the lowest pepsinogen I/II ratio and the highest seropositivity for H pylori. GA was found more frequently in patients positive for the APC mutation whereas no such a trend was observed in FGP.
Conclusions: In FAP, H pylori associated atrophic gastritis contributes negatively to FGP. It seems to contribute positively to GA, especially in patients with truncating APC gene mutation.
Keywords: familial adenomatous polyposis, fundic gland polyposis, gastric adenoma, atrophic gastritis, Helicobacter pylori
Familial adenomatous polyposis (FAP) or Gardner’s syndrome is an autosomal dominantly inherited disease characterised by innumerable adenomas in the large intestine and a high risk of colorectal cancer.1 The majority of patients with FAP also develop gastroduodenal lesions, including fundic gland polyposis in the gastric body and multiple adenomas in the antrum and/or duodenum.2–14 Although some genetic abnormalities specific to upper gastrointestinal neoplasia have been suggested within the adenomatous polyposis coli (APC) gene in animal experiments,15,16 little is known of the impact of APC gene mutation on gastric lesions in patients with FAP.
The degree of atrophic gastritis and Helicobacter pylori infection has been shown to be associated with the development of some tumours and tumour-like lesions of the stomach in the general population. Adenocarcinoma of the intestinal-type, adenoma, and hyperplastic polyp are considered to occur in the atrophic gastric mucosa with H pylori infection,17–19 while diffuse-type adenocarcinoma and fundic gland polyps tend to arise in the non-atrophic gastric mucosa with or without H pylori infection.19,20 While the risk of gastric cancer in FAP remains controversial,14,21,22 the high incidence of gastric cancer in Japanese patients with the disease compared with that in Western areas may be explained by H pylori infection.19,23–25
While genetic alteration is believed to be the major predisposing factor for the development of upper intestinal lesions in FAP, the role of H pylori infection and atrophic gastritis in the pathogenesis of gastric lesions has not been determined. In the current study, we evaluated H pylori status and the degree of gastric mucosal atrophy in relation to gastric lesions in FAP patients.
METHODS
Subjects
Between November 1997 and December 1999, 31 patients from 24 families, all of whom had an established diagnosis of FAP and underwent upper gastrointestinal endoscopy, were enrolled in the current study. Patients included 19 men and 12 women, ranging in age from 10 to 72 years (mean 40 years). Time intervals between the initial diagnosis of FAP and the current endoscopic investigations ranged from 0 to 25 years (mean 9.7). None of the patients had developed carcinoma of the stomach or duodenum. Twenty four patients had undergone a total or subtotal colectomy, and 13 patients had colorectal cancer. Prophylactic total colectomy was recommended in the remaining seven patients. Gardner’s stigmata, such as bone and soft tissue tumours, were found in 25 patients. Informed consent was obtained from each patient regarding the objective and protocol of the study.
Endoscopy and biopsy
Upper endoscopy was performed by an experienced endoscopist (MI) with a side viewing video endoscope (JF-200 or JF-230; Olympus, Tokyo, Japan) using the sprayed dye (0.2% indigo carmine) technique to confirm the presence of minute polyps. Whenever polypoid and/or depressed lesions were recognised in each of the gastric body and/or antrum, biopsy specimens were obtained from the largest and/or the irregularly shaped lesions in each site. In addition, biopsy specimens were also taken from the five points of the normal appearing mucosa at the lesser and greater curvature of the antrum and corpus and at the incisura angularis, as recommended in the updated Sydney system.26 The total number of biopsy samples ranged from 5 to 9 (mean 6.9).
Histological assessment of background gastric mucosa
The biopsy specimens taken from the five sites of the normal appearing gastric mucosa were fixed in 10% formalin, embedded in paraffin, and routinely stained with haematoxylin and eosin. In each biopsy specimen, the degree of glandular atrophy was graded as normal (grade 0), mild (grade 1), moderate (grade 2), or marked (grade 3) by one observer (SN), according to the updated Sydney system.26 The specimen from the incisura angularis was treated as an additional antral specimen.26 The highest grade among three or two samples was chosen for the individual histological score at the antrum and corpus. H pylori status was also assessed by histological examination of the five biopsy specimens after being stained using the modified Giemsa stain and immunostained with polyclonal rabbit anti-H pylori antibody B471 (Dako, Glostrup, Denmark).27
Serological assay of H pylori antibody and levels of pepsinogens
Serum IgG antibodies to H pylori were measured by an enzyme linked immunoadsorbent assay using the high molecular weight cell associated protein immunoassay kit (Kyowa Medex, Tokyo, Japan),28 and patients whose antibody titre was higher than the cut off value of 2.2 were regarded as positive. Serum levels of pepsinogen (PG) I and PG II were measured by a modified radioimmunoassay method using Riabead Kits (Dainabot, Tokyo, Japan), as described elsewhere.29 The PG I/PG II ratio was calculated and used as a serological marker for the degree of atrophic change in the gastric mucosa.30,31
APC gene analysis
Genomic DNA was isolated from peripheral blood using a standard protocol.32 Mutation of the APC gene was investigated by polymerase chain reaction based single strand conformation polymorphism and direct sequencing, as reported previously.33 Based on the study of Enomoto and colleagues,34 patients in whom the APC mutation was positive were subclassified as either the proximal mutation group, who had a mutation at a proximal region up to codon 416 in exon 9, or the distal mutation group, with the mutation at a more distal region.
Statistical analysis
Numerical data are given as mean (SD) unless otherwise stated. Statistical differences were evaluated using Fisher’s exact probability test, χ2 test, Mann-Whitney U test, or Kruskal-Wallis test. A p value less than 0.05 for each test was regarded as statistically significant.
RESULTS
Prevalence of gastric lesions and APC gene mutation
Of the 31 patients with FAP, gastric lesions were detected in 23 patients (74%) while duodenal adenoma was detected in 27 patients (87%). Fundic gland polyposis (FGP) was found in 16 patients (52%) and gastric adenoma (GA) in 12 patients (39%). FGP positive patients were younger than FGP negative patients (33.4 (14.4) v 46.5 (16.4) years; p<0.05) while ages did not differ between GA positive and negative patients (table 1 ▶).
Table 1.
Relationship between the presence of gastric lesions and mutation of the APC gene in patients with familial adenomatous polyposis
| Fundic gland polyposis | Gastric adenoma | |||||
| Positive (n=16) | Negative (n=15) | p Value | Positive (n=12) | Negative (n=19) | p Value | |
| Age (y) | 33.4 (14.4) | 46.5 (16.4) | <0.05* | 41.8 (3.4) | 38.5 (18.4) | 0.43* |
| Sex (M/F) | 8/8 | 11/4 | 0.17† | 6/6 | 13/6 | 0.26† |
| APC gene mutation | ||||||
| Positive (proximal/distal) | 12 (4/8) | 10 (2/8)‡ | 0.57† | 12 (2/10) | 10 (4/6)‡ | <0.01† |
| Negative | 4 | 4‡ | 0 | 8‡ | ||
*Mann-Whitney U test.
†Fisher’s exact probability test.
‡Data not available in one patient.
Data on mutations of the APC gene were available in 30 patients. In the remaining one patient, sufficient amount of genomic DNA could not be obtained. The APC germline mutations were identified in 22 of 30 patients (73%). Eight patients (27%) were considered to be negative for the APC mutation. Of the 22 patients positive for the APC mutation, six were subclassified into the proximal mutation group and 16 into the distal mutation group.34
The relationship between the presence of gastric lesions and mutation of the APC gene is shown in table 1 ▶. The rate of positivity of the APC gene mutation did not differ between patients with FGP (12/16, 75%) and those without FGP (10/14, 71%). Conversely, all patients with GA had the APC mutation while the mutation was found in only 10 of 18 patients (56%) without GA (p<0.01). Among patients positive for the APC mutation, the proximal mutation group had GA less frequently (2/6, 33%) than the distal mutation group (10/16, 63%) but the difference was not statistically significant (p=0.23).
Positivity of H pylori antibody, PG I and PG II, and histological atrophy score
Serologically, H pylori infection was positive in 12 of 31 patients (39%) with FAP. In each patient, the serological result was concordant with that determined by histology and/or immunohistochemistry. Neither age nor sex (male/female ratio) was different between the H pylori positive and negative patients. Table 2 ▶ summarises the relationships between gastric lesions and the serological/histological results.
Table 2.
Relationship between the presence of gastric lesions and Helicobacter pylori positivity and the degree of mucosal atrophy in patients with familial adenomatous polyposis
| Fundic gland polyposis | Gastric adenoma | |||||
| Positive (n=16) | Negative (n=15) | p Value | Positive (n=12) | Negative (n=19) | p Value | |
| H pylori positive cases | 2 (13%) | 10 (67%) | <0.005* | 7 (58%) | 5 (26%) | 0.08* |
| PG I (ng/ml) | 46.3 (14.2) | 44.9 (19.5) | 0.50† | 42.0 (20.6) | 48.0 (13.9) | 0.16† |
| PG II (ng/ml) | 9.2 (3.7) | 17.5 (12.3) | 0.11† | 14.6 (8.9) | 12.3 (10.4) | 0.39† |
| PG I/PG II ratio | 5.4 (1.6) | 3.4 (1.7) | <0.005† | 3.3 (1.2) | 5.2 (2.0) | <0.01† |
| Histological atrophy score | ||||||
| Antrum | 0.7 (0.7) | 1.5 (0.7) | <0.005† | 1.4 (1.0) | 0.9 (0.7) | 0.13† |
| Corpus | 0.2 (0.4) | 1.0 (0.8) | <0.005† | 0.9 (0.8) | 0.4 (0.6) | <0.05† |
PG, pepsinogen.
*Fisher’s exact probability test.
†Mann-Whitney U test.
The prevalence of H pylori seropositivity in patients with FGP (13%) was significantly lower than in those without FGP (67%) (p<0.005). Although serum levels of PG I and PG II did not differ between the two groups, the PG I/PG II ratio was significantly higher in FGP positive (5.4 (1.6)) than in FGP negative (3.4 (1.7)) patients (p<0.005). The histological score for glandular atrophy was lower in patients with FGP than in those without FGP at both the antrum and corpus (p<0.005).
H pylori positivity in patients with GA (58%) was higher than in those without GA (26%) but the difference was not statistically significant (p=0.08). Although serum levels of PG I and PG II did not differ between the two groups, the PG I/PG II ratio was significantly lower in GA positive (3.3 (1.2)) than in GA negative (5.2 (2.0)) patients (p<0.01). Histologically, patients with GA showed a higher degree of atrophy than those without GA, and the difference was statistically significant at the corpus (p<0.05) but not at the antrum (p=0.13). Among 12 patients with GA, the PG I/PG II ratio in H pylori positive patients (n=7) was significantly lower than in H pylori negative patients (n=5) (2.7 (1.2) v 4.0 (0.8); p<0.05).
H pylori status and PG I/PG II ratio in patients positive for APC gene mutation
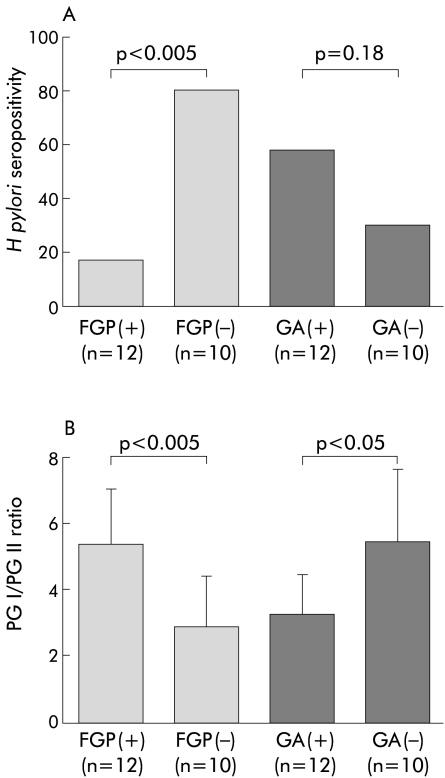
The relationship between H pylori status and gastric lesions in 22 patients positive for the APC gene mutation is demonstrated in fig 1A ▶. The rate of H pylori seropositivity in patients positive for the APC mutation with FGP (2/12, 17%) was significantly lower than in those without FGP (8/10, 80%) (p<0.005). H pylori positivity in patients positive for the APC mutation with GA (7/12, 58%) was higher than in those without GA (3/10, 30%) but the difference was not statistically significant.
Figure 1.
Helicobacter pylori seropositivity (A) and serum pepsinogen (PG) I/PG II ratio (B) in 22 patients positive for the adenomatous polyposis coli (APC) gene mutation in relation to gastric lesions. (A) The rate of H pylori seropositivity in patients positive for the APC mutation with fundic gland polyposis (FGP) (17%) was significantly lower than in those without FGP (30%) (Fisher’s exact probability test, p<0.005). H pylori positivity in patients positive for the APC mutation with gastric adenoma (GA) (58%) was higher than in those without GA (30%) but the difference was not statistically significant (p=0.18). (B) PG I/PG II ratio in patients positive for the APC mutation with FGP was significantly higher than in those without FGP (Mann-Whitney U test, p<0.005) while the ratio in those with GA was significantly lower than those without GA (p<0.05).
Figure 1B ▶ shows the relationship between the PG I/PG II ratio and gastric lesions in patients positive for the APC mutation. The PG I/PG II ratio in patients positive for the APC mutation with FGP (5.4 (1.6)) was significantly higher than in those without FGP (2.9 (1.5)) (p<0.005) while the ratio in those with GA (3.3 (1.2)) was significantly lower than in those without GA (5.4 (2.2)) (p<0.05).
Correlation of the type of gastric lesion with H pylori status and mucosal atrophy
Based on gastric lesions, patients were further divided into four groups as follows: 11 patients with FGP alone, seven patients with GA alone, five patients with both FGP and GA (FGP+GA group), and eight patients without gastric lesions (negative group). Table 3 ▶ shows a comparison of the four groups.
Table 3.
Comparison of the four groups by the presence or absence of gastric lesions in patients with familial adenomatous polyposis
| FGP alone (n=11) | FGP+GA (n=5) | GA alone (n=7) | Negative (n=8) | p Value | |
| Age (y) | 33.7 (15.3) | 32.8 (14.0) | 48.3 (9.2) | 45.0 (21.4) | 0.14* |
| Sex (M/F) | 6/5 | 2/3 | 4/3 | 7/1 | 0.27† |
| H pylori positive cases | 0 | 2 (40%) | 5 (71%) | 5 (63%) | <0.01† |
| PG I/PG II ratio | 6.0 (1.5) | 4.2 (1.0) | 2.6 (0.9) | 4.1 (2.0) | <0.005* |
| Histological atrophy score | |||||
| Antrum | 0.6 (0.7) | 0.8 (0.8) | 1.9 (0.9) | 1.3 (0.5) | <0.05* |
| Corpus | 0.1 (0.3) | 0.4 (0.5) | 1.3 (0.8) | 0.8 (0.7) | <0.05* |
FGP, fundic gland polyposis; GA, gastric adenoma; PG, pepsinogen.
*Kruskal-Wallis test.
†χ2 test.
Neither age nor the sex of the patients differed substantially among the four groups. The rate of H pylori seropositivity was lowest in the FGP alone group (0%) and highest in the GA alone group (71%). The FGP+GA group (40%) and the negative group (63%) showed an intermediate grade of H pylori positivity. A significant difference was found in H pylori positivity among the four groups (p<0.01).
The PG I/PG II ratio was highest in the FGP alone group (6.0 (1.5)) and lowest in the GA alone group (2.6 (0.9)). The FGP+GA group (4.2 (1.0)) and the negative group (4.1 (2.0)) showed an intermediate PG I/PG II ratio. There was a significant difference in the PG I/PG II ratio among the four groups (p<0.005). Significant differences in the ratio were also found between the FGP alone group and the FGP+GA group (p<0.05), and between the FGP+GA group and the GA alone group (p<0.05). The histological score for mucosal atrophy was significantly different among the four groups at both the antrum and corpus.
DISCUSSION
Upper gastrointestinal polyps have been reported in 46–100% of patients with FAP.2–14 Whereas duodenal adenoma is the most common lesion with a prevalence ranging from 40% to 92%, the prevalence of GA is 2–50% while that of FGP is 26–67%. In our study, gastric lesions were observed in 74% of FAP patients, and the prevalences of GA and FGP were 39% and 52%, respectively. These results are comparable with those reported in previous studies.
In the current study, we demonstrated that FGP and GA were closely associated with the degree of atrophic gastritis and H pylori status in FAP patients. FAP patients with FGP had a lower degree of atrophy or a higher PG I/PG II ratio and less frequent H pylori infections (table 2 ▶), which may be associated with the fact that FGP tended to be detected in younger FAP patients (table 1 ▶).7 Similar results in non-FAP patients have been described in several studies.20,35 Haruma and colleagues20 demonstrated that FGP tends to arise from the non-atrophic gastric mucosa, in contrast with the foveolar hyperplastic polyps. Sakai and colleagues35 reported H pylori infection in only three (4%) of 84 non-FAP patients with FGP. A recent abstract by Lakshman and colleagues36 showed that H pylori infection was not detected in any of 64 FAP patients with FGP. In addition, our gene analysis results suggested that the development of FGP is independent of the truncating APC germline mutation (table 1 ▶).34
Conversely, FAP patients with GA showed a higher degree of atrophy than those without GA (table 2 ▶). These results seem to be consistent with previously published data in non-FAP patients18,37,38; GA has been reported to develop in patients with severely atrophic gastritis18,38 or those with a low PG I/PG II ratio.37 Such mucosal atrophy in non-FAP patients with GA is generally considered to be induced by H pylori infection.39
In our study however, H pylori was only detected in 58% of FAP patients with GA (table 2 ▶). Our H pylori negative patients with GA showed a significantly higher PG I/PG II ratio than in H pylori positive patients with GA (4.0 (0.8) v 2.7 (1.2); p<0.05). Conversely, all of our patients with GA harboured the detectable APC gene mutation in contrast with those without GA (100% v 56%; table 1 ▶). These results suggest that the genesis of GA in H pylori negative patients may be directly associated with genetic abnormalities, as seen in adenomatosis in the colorectum or duodenum.34,40–42 Toyooka and colleagues41 detected somatic mutations in the APC gene in 26 of 29 (90%) GA in FAP while only three of 11 (27%) FGP had somatic mutations. Enomoto and colleagues34 showed that GAs were less frequently found in FAP patients with a germline mutation at the proximal region of the APC gene up to codon 416 (exon 9) than in those with a more distal mutation; however, no such relationship was observed for FGP. Our finding that the proximal mutation group had GA less frequently than the distal mutation group (33% v 63%) thus closely correlated with their data.34 These observations suggest that detectable mutations in the central region of the APC gene may be associated with the development of GA in FAP.
In our study, five of 31 patients (16%) with FAP had both FGP and GA (FGP+GA group). Interestingly, patients in the FGP+GA group were regarded to have an intermediate grade of mucosal atrophy determined by the PG I/PG II ratio, histological scores, and H pylori seropositivity compared with the FGP alone group or the GA alone group (table 3 ▶). As a result, patients in the FGP+GA group were considered to have a mildly to moderately atrophic gastric mucosa. The precise mechanisms of synchronous occurrence of the two lesions in the FAP patients remain unclear. We speculate that FGP develops in the non-atrophic gastric mucosa without H pylori infection in young FAP patients and thereafter GA arises during progression of antral mucosal atrophy, partly due to H pylori infection as well as other genetic or environmental factors. Long term follow up studies are necessary however to confirm this hypothesis.
In conclusion, the occurrence of gastric lesions in FAP was found to be closely associated with the degree of gastric mucosal atrophy and H pylori status. FGP is considered to arise in the non-atrophic mucosa without H pylori infection, irrespective of the APC gene mutation. Conversely, GA tends to occur in the atrophic mucosa with H pylori infection, and detectable mutations in the central region of the APC gene may be associated with its development. The necessity of eradicating H pylori still needs to be elucidated. Further investigations in a large number of patients, including genetic analyses, are therefore called for to clarify the mechanism of the development of gastric lesions in FAP.
Acknowledgments
The authors thank Brian Quinn for editing the manuscript. A preliminary report of this study was presented at the 59th annual meeting of Japan Gastroenterological Endoscopy Society, Kyoto, Japan, 30 May 2000.
Abbreviations
FAP, familial adenomatous polyposis
APC, adenomatous polyposis coli
PG, pepsinogen
FGP, fundic gland polyposis
GA, gastric adenoma
REFERENCES
- 1.Bussey HJR. Familial polyposis coli: familial studies, histopathology, differential diagnosis and results of treatment. Baltimore: Johns Hopkins University Press, 1975.
- 2.Yao T, Iida M, Ohsato K, et al. Duodenal lesions in familial polyposis of the colon. Gastroenterology 1977;73:1086–92. [PubMed] [Google Scholar]
- 3.Watanabe H, Enjoji M, Yao T, et al. Gastric lesions in familial adenomatosis coli: their incidence and histologic analysis. Hum Pathol 1978;9:269–83. [DOI] [PubMed] [Google Scholar]
- 4.Ranzi T, Castagnone D, Velio P, et al. Gastric and duodenal polyps in familial polyposis coli. Gut 1981;22:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Järvinen H, Nyberg M, Peltokallio P. Upper gastrointestinal tract polyps in familial adenomatosis coli. Gut 1983;24:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burt RW, Berenson MM, Lee RG, et al. Upper gastrointestinal polyps in Gardner’s syndrome. Gastroenterology 1984;86:295–301. [PubMed] [Google Scholar]
- 7.Iida M, Yao T, Itoh H, et al. Natural history of fundic gland polyposis in patients with familial adenomatosis coli/Gardner’s syndrome. Gastroenterology 1985;89:1021–5. [DOI] [PubMed] [Google Scholar]
- 8.Bülow S, Lauritsen KB, Johansen A, et al. Gastroduodenal polyps in familial polyposis coli. Dis Colon Rectum 1985;28:90–3. [DOI] [PubMed] [Google Scholar]
- 9.Sarre RG, Frost AG, Jagelman DG, et al. Gastric and duodenal polyps in familial adenomatous polyposis: a prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut 1987;28:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iida M, Yao T, Itoh H, et al. Natural history of gastric adenomas in patients with familial adenomatosis coli/Gardner’s syndrome. Cancer 1988;61:605–11. [DOI] [PubMed] [Google Scholar]
- 11.Domizio P, Talbot IC, Spigelman AD, et al. Upper gastrointestinal pathology in familial adenomatous polyposis: results from a prospective study of 102 patients. J Clin Pathol 1990;43:738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church JM, McGannon E, Hull-Boiner S, et al. Gastroduodenal polyps in patients with familial adenomatous polyposis. Dis Colon Rectum 1992;35:1170–3. [DOI] [PubMed] [Google Scholar]
- 13.Sawada T, Muto T. Familial adenomatous polyposis: should patients undergo surveillance of the upper gastrointestinal tract? Endoscopy 1995;27:6–11. [DOI] [PubMed] [Google Scholar]
- 14.Wallace MH, Phillips RKS. Upper gastrointestinal disease in patients with familial adenomatous polyposis. Br J Surg 1998;85:742–50. [DOI] [PubMed] [Google Scholar]
- 15.Fodde R, Edelmann W, Yang K, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA 1994;91:8969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smits R, van der Houven van Oordt W, Luz A, et al. Apc1638N: a mouse model for familial adenomatous polyposis-associated desmoid tumors and cutaneous cysts. Gastroenterology 1998;114:275–83. [DOI] [PubMed] [Google Scholar]
- 17.Correa P. A human model of gastric carcinogenesis. Cancer Res 1988;48:3554–60. [PubMed] [Google Scholar]
- 18.Nakano H, Persson B, Slezak P. Study of the gastric mucosal background in patients with gastric polyps. Gastrointest Endosc 1990;36:39–42. [DOI] [PubMed] [Google Scholar]
- 19.Tabata H, Fuchigami T, Kobayashi H, et al. Helicobacter pylori and mucosal atrophy in patients with gastric cancer: a special study regarding the methods for detecting Helicobacter pylori. Dig Dis Sci 1999;44:2027–34. [DOI] [PubMed] [Google Scholar]
- 20.Haruma K, Sumii K, Yoshihara M, et al. Gastric mucosa in female patients with fundic glandular polyps. J Clin Gastroenterol 1991;13:565–9. [DOI] [PubMed] [Google Scholar]
- 21.Jagelman DG, DeCosse JJ, Bussey HJR. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet 1998;1:1149–51. [DOI] [PubMed] [Google Scholar]
- 22.Offerhaus GJA, Giardiello FM, Krush AJ, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology 1992;102:1980–2. [DOI] [PubMed] [Google Scholar]
- 23.International Agency for Research of Cancer Monographs with Helicobacter pylori, vol 61. Lyon: IARC; 1994:177–240.
- 24.Huang JQ, Sridhar S, Chen Y, et al. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998;114:1169–79. [DOI] [PubMed] [Google Scholar]
- 25.Yamagata H, Kiyohara Y, Aoyagi K, et al. Impact of Helicobacter pylori infection on gastric cancer incidence in a general Japanese population: the Hisayama Study. Arch Intern Med 2000;160:1962–8. [DOI] [PubMed] [Google Scholar]
- 26.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura S, Yao T, Aoyagi K, et al. Helicobacter pylori and primary gastric lymphoma: a histopathologic and immunohistochemical analysis of 237 patients. Cancer 1997;79:3–11. [PubMed] [Google Scholar]
- 28.Evans DJ Jr, Evans DG, Graham DY, et al. A sensitive and specific serologic test for detection of Campylobacter pylori infection. Gastroenterology 1989;96:1004–8. [DOI] [PubMed] [Google Scholar]
- 29.Ichinose M, Miki K, Furihata C, et al. Radioimmunoassay of serum group I and group II pepsinogens in normal controls and patients with various disorders. Clin Chim Acta 1982;126:183–91. [DOI] [PubMed] [Google Scholar]
- 30.Samloff IM, Varis K, Ihamaki T, et al. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology: a study in relatives of patients with pernicious anemia. Gastroenterology 1982;83:204–9. [PubMed] [Google Scholar]
- 31.Miki K, Ichinose M, Shimizu A, et al. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn 1987;22:133–41. [DOI] [PubMed] [Google Scholar]
- 32.Miller SA, Dykes DD, Polesky HF. A simple salting our procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991;66:589–600 [DOI] [PubMed] [Google Scholar]
- 34.Enomoto M, Konishi M, Iwama T, et al. The relationship between frequencies of extracolonic manifestations and the position of APC germline mutation in patients with familial adenomatous polyposis. Jpn J Clin Oncol 2000;30:82–8. [DOI] [PubMed] [Google Scholar]
- 35.Sakai N, Tatsuta M, Hirasawa R, et al. Low prevalence of Helicobacter pylori infection in patients with hamartomatous fundic polyps. Dig Dis Sci 1998;43:766–72. [DOI] [PubMed] [Google Scholar]
- 36.Lakshman V, Shah AN, Ryan CK, et al. The presence of dysplasia and carcinoma in gastric fundic gland polyps in patients with familial adenomatous polyposis. Gastroenterology 1997;122:A599. [Google Scholar]
- 37.Morson BC, Sobin LH, Grundmann E, et al. Precancerous conditions and epithelial dysplasia in the stomach. J Clin Pathol 1980;33:711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshihara M, Sumii K, Haruma K, et al. Correlation of ratio of serum pepsinogen I and II with prevalence of gastric cancer and adenoma in Japanese subjects. Am J Gastroenterol 1998;93:1090–6. [DOI] [PubMed] [Google Scholar]
- 39.Komoto K, Haruma K, Kamada T, et al. Helicobacter pylori infection and gastric neoplasia: correlations with histological gastritis and tumor histology. Am J Gastroenterol 1998;93:1271–6. [DOI] [PubMed] [Google Scholar]
- 40.Nagase H, Miyoshi Y, Horii A, et al. Correlation between the location of germ-line mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res 1992;52:4055–7. [PubMed] [Google Scholar]
- 41.Toyooka M, Konishi M, Kikuchi-Yanoshita R, et al. Somatic mutations of the adenomatous polyposis coli gene in gastroduodenal tumors from patients with familial adenomatous polyposis. Cancer Res 1995;55:3165–70. [PubMed] [Google Scholar]
- 42.Leggett BA, Young JP, Biden K, et al. Severe upper gastrointestinal polyposis associated with sparse colonic polyposis in a familial adenomatous polyposis family with an APC mutation at codon 1520. Gut 1997;41:518–21. [DOI] [PMC free article] [PubMed] [Google Scholar]