Abstract
Background: Meal ingestion is often associated with exacerbation of gastrointestinal symptoms in subjects with irritable bowel syndrome (IBS). Furthermore, recent preliminary data suggest that 5-hydroxytryptamine (5-HT) concentration in platelet poor plasma is elevated following meal ingestion in some subjects with diarrhoea predominant IBS (d-IBS) compared with healthy subjects, although it is not known whether this is related to postprandial symptomatology.
Aim: To expand on previous data by evaluating a larger number of subjects but also to assess plasma 5-hydroxyindole acetic acid (5-HIAA) concentrations, 5-HT turnover, platelet 5-HT stores, and any relationship to symptomatology.
Methods: We assessed platelet depleted plasma 5-HT and 5-HIAA concentrations for two hours (60 minute intervals) under fasting conditions, and then for a further four hours (30 minute intervals) after a standard carbohydrate meal (457 kcal), together with fasting platelet 5-HT concentrations in 39 female subjects with d-IBS (aged 19–52 years; mean age 33) and 20 healthy female volunteers (aged 20–46 years, mean age 28). IBS symptomatology, in particular abdominal pain and bloating, and urgency to defecate were assessed throughout the study
Results: When related to fasting levels, there was no statistically significant difference in postprandial plasma 5-HT concentrations between d-IBS and healthy subjects. However, when fasting levels were not taken into consideration, d-IBS subjects exhibited higher postprandial plasma 5-HT concentrations compared with healthy subjects (p=0.040). Furthermore, d-IBS subjects who exhibited postprandial symptomatology had higher levels of postprandial plasma 5-HT, whether assessed with respect to fasting baseline levels (p=0.069) or not (p=0.047), compared with d-IBS subjects who did not report postprandial symptomatology. This appeared to be associated with a concomitant increase in plasma 5-HIAA (p=0.161) but reduction in turnover (p=0.058). Lastly, d-IBS subjects had higher platelet concentrations of 5-HT than healthy subjects (p=0.009).
Conclusions: These data suggest that postprandial symptomatology may be associated with increased platelet depleted plasma 5-HT concentrations in female subjects with d-IBS. In addition, the presence of increased platelet stores of 5-HT may act as a useful marker for the diagnosis and management of d-IBS.
Keywords: 5-hydroxytryptamine, 5-hydroxyindole acetic acid, diarrhoea, irritable bowel syndrome
Many subjects with irritable bowel syndrome (IBS) report exacerbation of their symptoms, particularly abdominal pain and gas, following food ingestion.1,2 This appears to be especially associated with meals rich in carbohydrates and fat,1 as well as being more common in those subjects who are female and/or anxious.2
Although the pathogenesis of symptoms remains unclear, prolonged and exaggerated colonic motor responses following meal ingestion have been reported in subjects with IBS compared with healthy controls,3–5 especially in those reporting postprandial pain.6 Other studies have shown increased jejunal sensitivity to balloon distension after feeding7 and jejunal lipid infusion8 in subjects with IBS. A similar response was not reported with electrical stimulation however, suggesting that lipids selectively modulate the mechanosensory pathways.8
5-Hydroxytryptamine (5-HT), found predominantly within the gastrointestinal tract (80% of body 5-HT), both in enteroendocrine cells (95%) and enteric neurones (5%), is known to play a significant role in the control of gastrointestinal motility, sensation, and secretion.9,10 Released by neuronal, chemical, or mechanical stimulation, it acts via a variety of 5-HT receptors either directly, for example on the muscle or enterocytes themselves, or indirectly through, for example, vagal afferent fibres and enteric neurones to modulate gastrointestinal function. 5-HT found in the blood is almost entirely derived from the gastrointestinal tract11 where it is either rapidly taken up by platelets,12 which consequently contain the majority of 5-HT in peripheral blood, or is metabolised by the liver and kidney to its metabolite 5-hydroxyindole acetic acid (5-HIAA).13 Concentrations of 5-HT in platelet depleted plasma will therefore more accurately reflect transient changes in concentration whereas those in platelets might be more indicative of any change over time.
Pilot data have suggested that the concentration of 5-HT in platelet poor plasma appears to be higher in female subjects with diarrhoea predominant IBS (d-IBS) compared with healthy subjects under fed conditions,14 suggesting a possible role for 5-HT in postprandial symptoms and may be even the disordered motor and sensory function seen in some of these subjects. IBS symptomatology however was not assessed in these studies and the number of subjects was small. Furthermore, the groups were not sex matched (only one of the healthy subjects was female) and as recent data indicate that 5-HT turnover may be reduced in healthy females compared with males,15 this may have influenced their results.
The aim of this study was to measure platelet depleted plasma 5-HT and its metabolite 5-HIAA concentrations in a much larger group of female subjects with d-IBS and healthy subjects under both fasting and fed conditions, and to examine the relationship between postprandial IBS symptomatology and 5-HT/5-HIAA concentrations. In addition, as platelets possess an avid uptake mechanism to extract 5-HT from plasma,12 fasting platelet stores of 5-HT were assessed in both IBS and healthy subjects.
MATERIALS AND METHODS
Subjects
The study (protocol number S3B30007) was carried out on 39 female subjects with d-IBS, diagnosed according to the Rome II criteria16 (mean years since first diagnosis was 6.91 years), aged 19–52 years (mean 33.4), and 20 healthy female subjects aged 20–46 years (mean 28.2). No subject had coexistent disease and all had normal haematology, biochemistry, urinalysis, and sigmoidoscopy, together with a normal colonoscopy or barium enema if aged over 40 years. All healthy subjects had normal laboratory investigations (as above) and negative toxicology for substances of abuse. Subjects were excluded if they had: a history of gastrointestinal surgery (other than appendectomy, cholecystectomy, and hiatus hernia repair); gastrointestinal symptoms related to or exacerbated by consumption of milk or milk products; or were taking drugs that might modify either gastrointestinal function or the 5-HT system, such as analgesic medication, tranquillisers, or antidepressants. Subjects were excluded if they were pregnant, breast feeding, or hysterectomised, and had to be postpuberty and premenopausal. As there is evidence that steroid ovarian hormones might affect the 5-HT system,17 all subjects were studied during the luteal phase of the menstrual cycle (high progesterone and oestrogen) or while taking combined (non-phased) oestrogen/progesterone contraceptive medication. All medications and cigarette smoking were prohibited for 48 hours prior to the study while alcohol and caffeine containing drinks were stopped 24 hours before the study. All subjects drank below the recommended safe alcohol limit (<21 units/week), smoked <5 cigarettes per day, and had not participated in a clinical trial of any drug within the previous 30 days.
The study was powered using data (median and range of peak plasma 5-HT values) obtained from Bearcroft and colleagues14 which suggested that there was considerably more variation in platelet poor plasma 5-HT levels in IBS subjects compared with healthy subjects, that these data were skewed, and as such potentially not normally distributed. Taking these factors into account and that any estimate of standard deviation using range data assumes normality, a conservative estimate of a standard deviation of 440 nmol/l was used. Using this estimate in the sample size calculation with 80% power, 39 subjects were required per group to detect a difference of 280 nmol/l between IBS and healthy subjects at a statistically significant level of 5%. However, as the variability in peak 5-HT values is considerably lower in healthy subjects, it was considered reasonable to restrict the number of these to 2:1 in favour of IBS subjects. Thus we aimed to recruit approximately 39 female d-IBS subjects and 19 female healthy subjects. It was also considered that loss of power resulting from the unequal allocation was adequately compensated for by using the conservative estimate of the standard deviation and as such the design was sufficiently powered to evaluate the aims of the study.
The study was approved by South Manchester Medical Research Ethics Committee and all subjects gave written informed consent.
Study design
After an overnight fast, subjects attended the Clinical Investigations Unit and an arm vein was cannulated. Blood (9 ml) was taken via vacutainer containing 0.3 ml citrate solution at 0 hours for platelet count and 5-HT/5-HIAA analysis. Further 5 ml blood samples for 5-HT and 5-HIAA analysis were taken at hourly intervals for two hours under fasting conditions and at half hourly intervals for a further four hours following ingestion of a standard carbohydrate rich meal consisting of 200 g spaghetti (Heinz, Stockley Park, Uxbridge, UK), two medium slices of toast, a jam and fresh cream scone (Marks and Spencer, London, UK), and 200 ml water (totalling 65.5 g carbohydrate, 12 g protein, 16 g fat, calorie content of 457 kcal) which was consumed within 10 minutes.
Symptomatology was assessed on attendance at the laboratory with the question “Is your IBS active at the moment?” In addition, at hourly intervals (0, 1, 2, 3 hours, etc) throughout the study, questions targeting the presence and severity of abdominal pain/discomfort, bloating, and bowel urgency were asked, for example “In the past hour have you experienced abdominal pain/discomfort?”. If the subjects reported “yes”, they were then asked to grade the severity of that symptom using the scale 1=mild, 2=moderate, 3=intense, and 4=severe. A worsening of symptoms with meal ingestion was defined as an increase in symptom score of at least 1.
Analysis of platelet depleted plasma concentration of 5-HT and 5-HIAA
Collected blood (t=−1 to 4 hours post meal) was transferred to tubes containing 0.5 ml of 3.12% trisodium citrate and centrifuged (room temperature) twice to ensure no platelet contamination of supernatant; initially at 2500 rpm for 10 minutes and then at 4000 rpm for a further 10 minutes. Platelet depleted plasma was aspirated and duplicate samples stored at −70°C for later batch analysis. 5-HT and 5-HIAA concentrations were measured in duplicate using high performance liquid chromatography with fluorometric detection.18–20 All samples were analysed blind to subject status.
The sensitivity of the high performance liquid chromatography system for the detection of 5-HT and 5-HIAA (based on 50 μl injection volume) was at least 5 nmol/l, with a signal to noise ratio of greater than 10 and a coefficient of variation (CV) <20%. The intra-assay CVs for controls for 30.10 nmol/l and 55.66 nmol/l of 5-HT were 4.6% and 4.3%, respectively, and the inter-assay CVs at the same levels of 5-HT were 6.2% and 6.4%. The intra-assay CVs for controls for 26.15 nmol/l and 52.82 nmol/l of 5-HIAA were 5.2% and 4.9%, respectively, and the inter-assay CVs were 6.7% and 6.3%.
To ensure that there had been no platelet activation and/or leakage due to errors in blood collection and preparation, thromboxane β2 (a platelet activation marker) was measured in platelet depleted plasma using a commercial ELISA method (Amersham Life Science Ltd, Bucks, UK).21 The β-thromboglobulin detection limit was 0.1 fg per well which equates to less than 0.1% of platelet activation.
Analysis of platelet 5-HT levels
Blood collected at t=0 was transferred to a tube containing 0.9 ml of 3.13% trisodium citrate and initially spun only once at 900 rpm for five minutes. One aliquot of aspirated platelet rich plasma was then used to assess platelet count (Advia Centaur Analyser; Bayer Ltd, Berks, UK) while additional duplicate samples were used for 5-HT analysis of platelet rich plasma, and following further centrifugation (as above) for analysis of platelet depleted plasma 5-HT/5-HIAA concentrations.
Analysis of sex hormone levels
Plasma concentrations of the female sex hormones oestrogen, progesterone, follicular stimulating hormone, luteinising hormone, and combined oral contraceptive pill component drugs were assessed at t=0 by a commercial immunoassay system on an Advia Centaur Analyser (Bayer Ltd).
Data and statistical analysis
The following end points were analysed for 5-HT and 5-HIAA platelet depleted plasma concentrations:
Fasting concentration, calculated as the average of the preprandial measurements.
Ratio to fasting baseline area under the postprandial concentration/time curve. This was calculated as the area under the postprandial concentration curve (AUC) versus time, divided by fasting concentration multiplied by four.
Peak postprandial concentration.
Other end points included platelet 5-HT concentration, area under the postprandial 5-HT concentration/time curve (not referenced to fasting baseline), fasting 5-HT turnover, and ratio to baseline area under the postprandial 5-HT turnover/time curve.
Linear models were fitted to the log transformation of the above variables. All models included terms for subject group and age, and analysis of ratio to fasting baseline AUC and peak postprandial concentration also included a term for fasting concentration. The effect of the hormones noted above was assessed and included in the model where appropriate. All models were checked for the validity of model assumptions.
In addition, the time to peak postprandial concentration was analysed non-parametrically for both platelet depleted plasma 5-HT and 5-HIAA using a Wilcoxon’s rank sum test.
RESULTS
Symptomatology
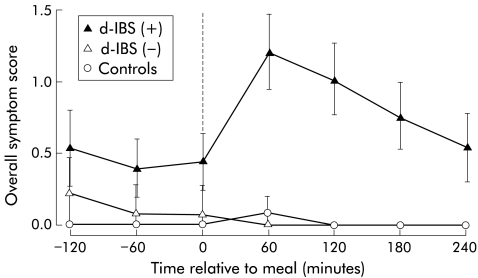
All d-IBS subjects reported that their IBS was active at the time of investigation, with 78% reporting a bowel frequency of 3–5 per day and 22% a bowel frequency of >5 per day. In addition, 94% of patients reported a history of loose to watery stools and 89% a frequent sensation of urgency before defecation. On the day of the study, 22 (56%) patients reported symptoms immediately before and 31 (79%) reported symptoms following ingestion of the meal. Of the 31 d-IBS subjects who reported symptoms postprandially, 28 (90%) exhibited worsening of symptoms compared with their pre-meal status, with symptoms peaking approximately one hour after the meal and remaining elevated for over three hours (fig 1 ▶). In contrast, none of the healthy subjects reported any symptoms before ingestion of the standard meal, and only two reported symptoms postprandially (fig 1 ▶).
Figure 1.
Profile of overall symptom score with respect to meal ingestion (t=0) in female diarrhoea predominant irritable bowel syndrome (d-IBS) subjects with (+) and without (−) postprandial symptoms, and in healthy female subjects. Data are means and 95% confidence interval.
Platelet depleted plasma 5-HT concentrations
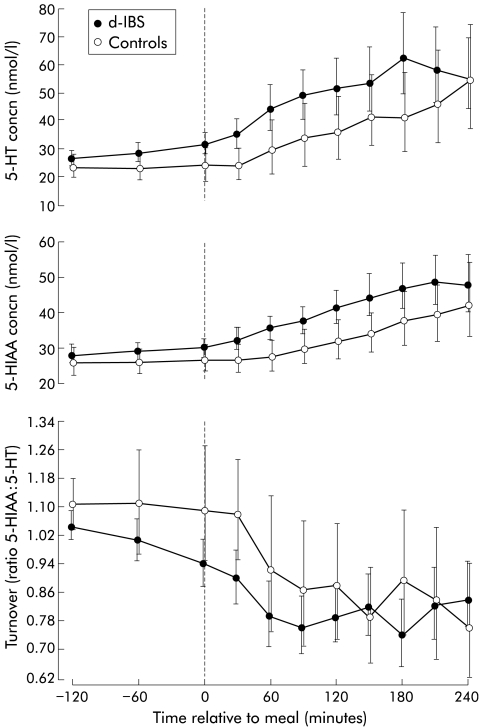
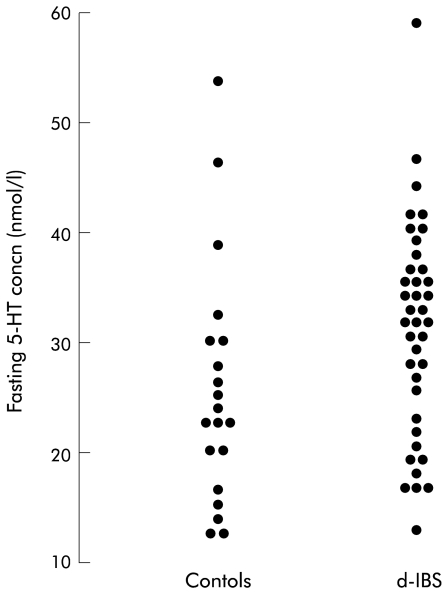
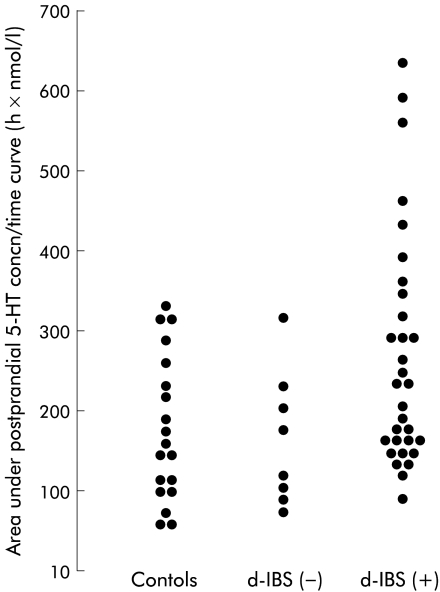
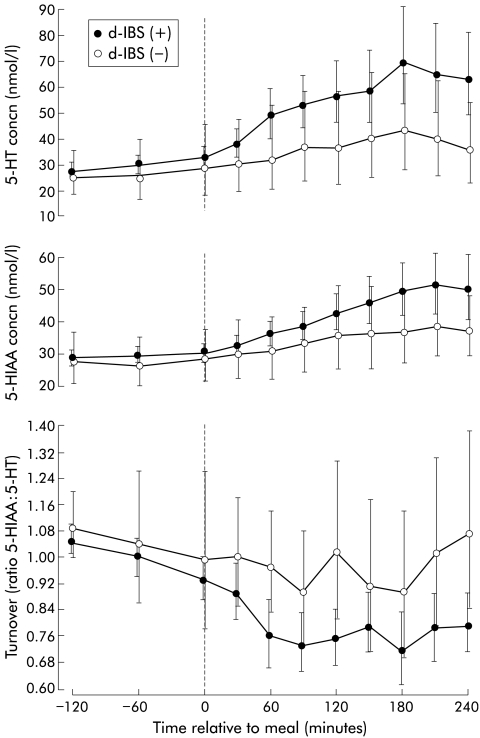
Under fasting conditions, the concentration of platelet depleted plasma 5-HT in d-IBS subjects was not significantly different from that of healthy subjects (table 1 ▶, fig 2 ▶), although as can be seen from fig 3 ▶ d-IBS subjects tended to have higher fasting concentrations. Ingestion of the meal increased the concentration of platelet depleted plasma 5-HT in both IBS and healthy subjects but there was no significant difference in the ratio to fasting baseline area under the postprandial concentration/time curve (AUC) between the two groups (fig 2 ▶, table 1 ▶). Assessment of the area under the postprandial 5-HT concentration/time curve only (not referenced to fasting baseline) however, showed that d-IBS subjects had higher postprandial concentrations than healthy subjects (d-IBS subjects: 211.3 h×nmol/l, adjusted geometric mean v healthy subjects: 151.1 h×nmol/l; p=0.040) (fig 4 ▶). Furthermore, the peak postprandial platelet depleted plasma 5-HT concentration occurred earlier in d-IBS patients compared with healthy subjects (IBS subjects: 158 (59) minutes (mean (SD)) v healthy subjects: 188 (70) minutes; p=0.041) and was associated with significantly more of the d-IBS subjects reaching a peak 5-HT concentration within the four hours post meal than in healthy subjects (34/39 v 10/19; p<0.004).
Table 1.
Comparison of platelet depleted plasma 5-hydroxytryptamine (5-HT) and 5-hydroxyindole acetic acid (5-HIAA) concentrations, and turnover, between female subjects with diarrhoea predominant irritable bowel syndrome (d-IBS) and healthy subjects
| d-IBS (n=39) | Healthy (n=20) | Ratio d-IBS to healthy | p Value | |
| 5-HT concn | ||||
| Fasting (nmol/l) | 28.00 | 25.45 | 1.10 (0.90,1.34) | 0.348 |
| Fed AUC | 1.73 | 1.51 | 1.15 (0.88,1.49) | 0.306 |
| Fed peak (nmol/l) | 76.11 | 87.81 | 0.87 (0.59,1.27) | 0.458 |
| 5-HIAA concn | ||||
| Fasting (nmol/l) | 28.24 | 27.20 | 1.04 (0.90,1.19) | 0.595 |
| Fed AUC | 1.40 | 1.19 | 1.18 (1.00,1.39) | 0.048 |
| Fed peak (nmol/l) | 53.50 | 47.23 | 1.13 (0.87,1.48) | 0.350 |
| Turnover (ratio 5-HIAA:5-HT) | ||||
| Fasting | 1.02 | 1.09 | 0.94 (0.85,1.05) | 0.260 |
| Fed AUC | 0.87 | 0.90 | 0.96 (0.86,1.08) | 0.512 |
| Platelet 5-HT (nmol/109 platelets) | ||||
| Fasting | 2.25 | 1.83 | 1.23 (1.05,1.43) | 0.009 |
Results are adjusted geometric means (95% confidence interval).
AUC, ratio to fasting baseline area under the postprandial concentration/time curve.
Figure 2.
Profiles of 5-hydroxytryptamine (5-HT) and 5-hydroxyindole acetic acid (5-HIAA) concentrations and turnover with respect to meal ingestion (t=0) in female subjects with diarrhoea predominant irritable bowel syndrome (d-IBS) and healthy female subjects. Data are geometric means and 95% confidence interval.
Figure 3.
Individual plots of values for fasting concentrations of platelet depleted plasma 5-hydroxytryptamine (5-HT) in female subjects with diarrhoea predominant irritable bowel syndrome (d-IBS), and healthy subjects.
Figure 4.
Individual plots of values for area under the postprandial 5-hydroxytryptamine (5-HT) concentration/time curve (not referenced to fasting levels) in female subjects with diarrhoea predominant irritable bowel syndrome (d-IBS) with (+) and without (−) postprandial symptoms and in healthy subjects.
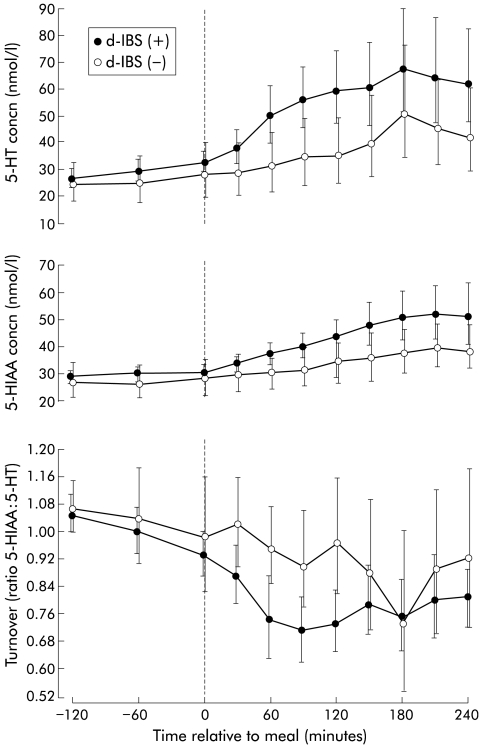
Subjects with d-IBS who reported symptoms following the meal tended to exhibit higher concentrations of postprandial platelet depleted plasma 5-HT relative to fasting concentrations compared with those who did not report postprandial symptoms (p=0.069) (fig 5 ▶, table 2 ▶). This was also seen when comparing the area under the postprandial plasma 5-HT concentration/time curves only (not referenced to fasting baseline) for d-IBS subjects with, compared with those without, postprandial symptoms (d-IBS subjects with symptoms: 227.2 h×nmol/l, adjusted geometric mean v d-IBS subjects without symptoms: 149.9 h×nmol/l; p=0.047) (fig 4 ▶). Likewise, the peak postprandial platelet depleted plasma 5-HT concentration tended to be higher in d-IBS subjects with symptoms compared with those without symptoms (p=0.079) (table 2 ▶). There was no difference in the time to postprandial plasma peak (d-IBS subjects with symptoms: 160 (61) minutes (mean (SD)) v d-IBS subjects without symptoms: 150 (53) minutes; p=0.659) or fasting concentrations of platelet depleted plasma 5-HT between d-IBS subjects who either did or did not go on to report postprandial symptoms (table 2 ▶). Similar data were obtained when comparing d-IBS subjects who either did or did not exhibit a worsening of symptoms following meal ingestion compared with their pre-meal status (fig 6 ▶).
Figure 5.
Profiles of 5-hydroxytryptamine (5-HT) and 5-hydroxyindole acetic acid (5-HIAA) concentrations and turnover with respect to meal ingestion (t=0) in female subjects with diarrhoea predominant irritable bowel syndrome (d-IBS) with (+) and without (−) postprandial symptoms. Data are geometric means and 95% confidence interval.
Table 2.
Comparison of platelet depleted plasma 5-hydroxytryptamine (5-HT) and 5-hydroxyindole acetic acid (5-HIAA) concentrations, and turnover, between female subjects with diarrhoea predominant irritable bowel syndrome (d-IBS) with and without postprandial (pp) symptoms
| With pp symptoms (n=31) | Without pp symptoms (n=8) | Ratio with to without pp symptoms | p Value | |
| 5-HT concn | ||||
| Fasting (nmol/l) | 29.20 | 28.57 | 1.02 (0.81,1.30) | 0.851 |
| Fed AUC | 1.78 | 1.26 | 1.41 (0.97,2.05) | 0.069 |
| Fed peak (nmol/l) | 87.59 | 54.58 | 1.61 (0.94,2.73) | 0.079 |
| 5-HIAA concn | ||||
| Fasting (nmol/l) | 28.87 | 28.87 | 1.00 (0.84,1.19) | 0.989 |
| Fed AUC | 1.43 | 1.21 | 1.18 (0.93,1.49) | 0.161 |
| Fed peak (nmol/l) | 57.32 | 44.77 | 1.28 (0.88,1.86) | 0.192 |
| Turnover (ratio 5-HIAA:5-HT) | ||||
| Fasting | 1.00 | 1.02 | 0.98 (0.88,1.09) | 0.664 |
| Fed AUC | 0.86 | 1.01 | 0.85 (0.73,1.01) | 0.058 |
| Platelet 5-HT (nmol/109 platelets) | ||||
| Fasting | 2.18 | 2.36 | 0.92 (0.76,1.13) | 0.416 |
Results are adjusted geometric means (95% confidence interval).
AUC, ratio to fasting baseline area under the postprandial concentration/time curve.
Figure 6.
Profiles of 5-hydroxytryptamine (5-HT) and 5-hydroxyindole acetic acid (5-HIAA) concentrations and turnover with respect to meal ingestion (t=0) in female subjects with diarrhoea predominant irritable bowel syndrome (d-IBS) with (+) and without (−) worsening of postprandial symptomatology. Data are geometric means and 95% confidence interval.
Platelet depleted plasma 5-HIAA concentrations
Under fasting conditions, the concentration of platelet depleted plasma 5-HIAA in d-IBS subjects was not significantly different from that of healthy subjects (fig 2 ▶, table 1 ▶). Ingestion of the standard meal was associated with higher concentrations of platelet depleted plasma 5-HIAA, relative to fasting, in d-IBS subjects compared with healthy subjects (postprandial:fasting AUC: p=0.048) but no difference in peak postprandial concentration (table 1 ▶) or time to this peak (d-IBS subjects: 184 (62) minutes (mean (SD)) v healthy subjects: 204 (50) minutes; p=0.209) between the two groups.
Although there was a tendency for d-IBS subjects with postprandial symptoms to have a higher peak platelet depleted plasma 5-HIAA concentration and an increased ratio to fasting baseline area under the postprandial 5-HIAA concentration/time curve (AUC) compared with d-IBS subjects without symptoms, these differences did not achieve statistical significance (fig 5 ▶, table 2 ▶). In addition, there was no statistically significant difference in either the time to postprandial 5-HIAA peak (d-IBS subjects with postprandial symptoms: 183 (66) minutes (mean (SD)) v d-IBS subjects without symptoms: 188 (45) minutes; p=0.830) or fasting concentrations of platelet depleted plasma 5-HIAA (fig 5 ▶, table 2 ▶) between d-IBS subjects who either did or did not report postprandial symptoms or between d-IBS subjects who either did or did not report worsening of symptoms following meal ingestion compared with pre-meal status (fig 6 ▶).
5-HT turnover (ratio 5-HIAA: 5-HT)
There was no significant difference in 5-HT turnover under fasting and fed conditions between d-IBS and healthy subjects (fig 2 ▶, table 1 ▶). However, d-IBS subjects with postprandial symptoms appeared to have a lower turnover than those without symptoms, as measured by the ratio to fasting baseline area under the postprandial turnover curve(p=0.058) (fig 5 ▶, table 2 ▶). Again, similar data were obtained when comparing d-IBS subjects who either did or did not exhibit worsening of symptoms following meal ingestion compared with their pre-meal status (fig 6 ▶).
Platelet 5-HT concentration
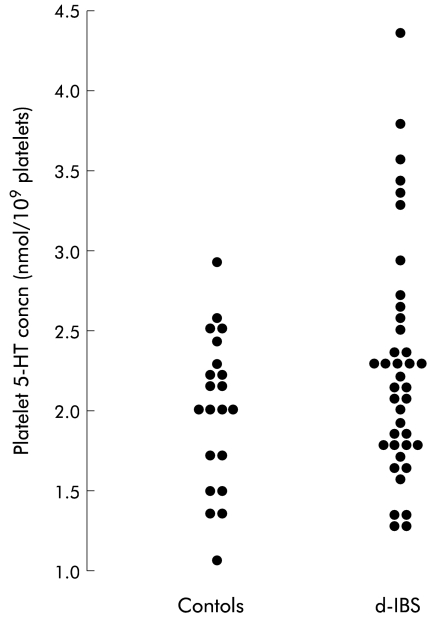
Subjects with d-IBS had significantly larger stores of platelet 5-HT compared with healthy subjects (p=0.009) (table 1 ▶, fig 7 ▶). However, there was no difference in stores between d-IBS subjects with and without postprandial symptoms (table 2 ▶). In addition, those patients with the highest platelet concentrations of 5-HT did not differ with respect to their clinical history or symptom severity on the day of the study to those patients who had lower platelet concentrations of 5-HT.
Figure 7.
Individual plots of values for platelet 5-hydroxytryptamine (5-HT) concentration in female subjects with diarrhoea predominant irritable bowel syndrome (d-IBS) and healthy subjects.
β-Thromboglobulin
β-Thromboglobulin was undetectable in all samples assessed.
DISCUSSION
Our results have shown for the first time that symptom exacerbation following meal ingestion in female subjects with d-IBS is associated with increased levels of plasma 5-HT, together with a reduction in 5-HT turnover. In addition, baseline platelet stores of 5-HT are elevated in female subjects with d-IBS compared with healthy subjects, supporting increased exposure of platelets to 5-HT in the systemic circulation.
It has previously been shown in normal healthy volunteers that a carbohydrate rich meal is a good stimulus for increased platelet poor plasma 5-HT concentrations.22 Our data confirm this observation, but in addition have shown that this is associated with a concomitant increase in its metabolite 5-HIAA but a reduction in 5-HT turnover overall (fig 2 ▶). This suggests that postprandially, 5-HT synthesis and/or its release from enteroendocrine cells, was in excess of its metabolism and uptake by both platelets and endothelia. It is unlikely that any increase in plasma osmolarity, glucose concentration, or circulating hormones following the meal caused leakage of 5-HT from platelets into the plasma, as no thromboxane β2 (an activation/leakage marker) was detected in any of the samples assessed. Lack of plasma thromboxane β2 together with the observation that both 5-HT and 5-HIAA concentrations were relatively similar and stable within subjects during the two hours prior to the meal, and similar to previous studies,15,18,19 also provides support that both the blood collection and preparation techniques were adequate.
In contrast with Bearcroft and colleagues14 who showed that postprandially plasma 5-HT concentrations were higher in female d-IBS subjects compared with healthy subjects, we did not find a similar increase when comparing postprandial plasma 5-HT concentrations relative to fasting concentrations. This is due to the different statistical approaches employed in analysing the data. Bearcroft and colleagues14 performed a non-parametric analysis on the area under the postprandial concentration/time curve only as they could not detect fasting 5-HT levels, whereas our study, which involved a large number of subjects, enabled us to use a linear modelling approach allowing us to take into account various factors (for example, fasting levels, sex hormones, etc) that might influence postprandial levels of platelet depleted plasma 5-HT. Using this approach we found that fasting levels significantly affected modelling of the postprandial area under the concentration/time curve, and this was equivalent to analysing the ratio to fasting baseline area under the curve. Consequently, any difference seen under postprandial conditions (fig 4 ▶) is influenced by differences in fasting levels (figs 2, 3 ▶ ▶). Indeed, when we analysed the area under the postprandial concentration/time curve without taking into account fasting levels (as was done by Bearcroft and colleagues14) we were able to reproduce their results (p=0.040). The elevated postprandial platelet depleted plasma 5-HT concentration found in d-IBS compared with healthy subjects was further supported by our observations that plasma 5-HIAA concentration was also significantly higher while turnover (ratio 5-HIAA:5-HT) was similar in d-IBS compared with healthy subjects. Furthermore, our new and potentially important observation that an excessive increase in plasma 5-HT appears to be related to expression of symptoms may have affected the overall comparison of d-IBS compared with healthy subjects, as not all d-IBS subjects reported symptoms.
Previous studies attempting to address the relationship between plasma 5-HT levels and functional gastrointestinal symptoms have been inconclusive, with one showing that only approximately 14% of subjects with severe gastrointestinal symptoms of a functional but poorly defined nature have elevated levels of plasma 5-HT23 and another showing only a slightly higher plasma 5-HT concentration in subjects with diarrhoea and abdominal pain.24 Both studies however were carried out under fasting conditions on a heterogeneous group of subjects with functional gastrointestinal disease and did not concurrently assess symptomatology and plasma 5-HT levels.
Measurement of platelet depleted plasma 5-HT concentration following a stimulus, such as a meal, may be important as metabolism and platelet uptake of 5-HT in plasma might make differentiation of plasma 5-HT levels between IBS and healthy subjects difficult under basal fasting conditions. Indeed, although our data may indicate that subjects with d-IBS tend to have higher concentrations of fasting platelet depleted plasma 5-HT, the study was not powered to be able to detect this difference. This is also in part supported by the fact that symptoms1,2 and abnormalities in motility, particularly those in the colon,25 appear to be more consistently reported under fed than fasting conditions in subjects with IBS, which may indicate that any abnormalities in plasma 5-HT concentrations are more readily observed under these conditions. Potential differences in small and large bowel motility between subjects with different functional gastrointestinal disorders, and between IBS subjects who have a diarrhoea versus a constipation predominant bowel habit,25–28 also implies that the precise nature of functional disorder under study needs to be carefully identified. Lastly, reports that age, sex, and sex hormone status may affect plasma 5-HT concentration1,15,17 indicates that these should also be matched when comparing different groups of subjects.
In addition to the observation that subjects with postprandial symptoms have elevated concentrations of plasma 5-HT, we have shown they also have a trend for increased plasma levels of its metabolite 5-HIAA compared with subjects without symptoms. Despite this however, turnover was significantly reduced suggesting that the synthesis and/or release from enteroendocrine cells of 5-HT was in excess of its metabolism and uptake by platelets and endothelia during the four hours after the meal. Whether these differences reflect increased synthesis and/or release of 5-HT which is in access of normal metabolism rates and/or reuptake, or whether there is an abnormality in the metabolism and/or reuptake mechanisms, cannot be elucidated from this study. Interestingly, platelet concentrations of 5-HT were significantly higher in the d-IBS group compared with the healthy group, supporting their increased but possibly intermittent exposure to excessive concentrations of plasma 5-HT. This increased platelet concentration of 5-HT could result from the presence of a serotonin transporter gene polymorphism that leads to greater 5-HT uptake as there is some evidence to suggest that serotonin transporter polymorphisms may be associated with different bowel habit types and influence drug treatment response in some subjects with IBS.29,30 However, our observation that fasting plasma 5-HT concentrations tend to be higher in d-IBS compared with healthy subjects may argue against this hypothesis. The apparent lack of relationship between platelet 5-HT concentration and clinical history and/or symptom severity on the day of study probably reflects the fact that platelet 5-HT levels are a consequence of a combination of factors, such as disease severity, carbohydrate/fat consumption, and/or stress/anxiety levels over a period of time. Given that platelet 5-HT concentration is entirely due to active transport of 5-HT from the circulation, as platelets cannot synthesise 5-HT, and that platelet levels may be a more reliable indicator of excessive but intermittent release of 5-HT than plasma levels, this suggests that platelet 5-HT concentrations may have a potential role to play as a marker in the diagnosis and management of d-IBS. This would be similar to the way glycosylated haemoglobin is used to reflect mean blood glucose concentration over a prolonged time period in patients with diabetes mellitus.31
Exactly why food on some but not other occasions leads to excessive increases in plasma 5-HT cannot be elucidated from the present study but it is of interest that this may occur in a manner that is consistent with the irregularity of postprandial symptoms. It is unlikely that this is related to differences in gastrointestinal transit time as the time to peak plasma 5-HT concentration and the shape of the concentration/time curves were similar between d-IBS subjects with and without postprandial symptoms. However, the whole d-IBS group who might be expected to have an accelerated transit compared with healthy subjects32 exhibited a peak in their plasma 5-HT concentration slightly before the healthy group, although the significantly greater area under the postprandial 5-HT concentration curve (not referenced to fasting) in patients compared with controls would still suggest that these differences in plasma 5-HT are not solely due to accelerated transit. A further possibility is that stress, which is known to exacerbate symptoms33 and motility,34 and release of 5-HT35 might have acted as a concomitant stimulus with food to release 5-HT from the enteroendocrine cells in d-IBS subjects presenting with than without postprandial symptoms. Alternatively, Elsenbruch and Orr36 have recently suggested that postprandial symptom severity is directly correlated with sympathetic dominance (secondary to vagal withdrawal) following meal ingestion in subjects with d-IBS, and as sympathetic activity releases 5-HT from enteroendocrine cells37 this might help explain the elevated platelet depleted plasma 5-HT levels in those patients with postprandial symptoms.
Whether the increased release of 5-HT into plasma leads to depletion of mucosal 5-HT in subjects with d-IBS still remains to be determined, but a recent study by Miwa and colleagues38 has suggested that subjects with constipation predominant IBS have elevated levels of mucosal 5-HT compared with subjects with d-IBS and healthy subjects, which might indicate an abnormality in enteroendocrine cell release of 5-HT to various stimuli. These investigators however while not assessing platelet depleted plasma 5-HT concentration did show a non-significant reduction in mucosal 5-HT in the small group of subjects with d-IBS compared with healthy subjects. Another recent study by Bose and colleagues39 (and personal communication) showed that colonic mucosal 5-HT concentrations were lower in d-IBS subjects compared with healthy subjects. This appeared to be associated with an increase in enteroendocrine cell numbers, which they suggested was a compensatory response of the gut to possible excessive release of 5-HT. Further studies addressing both mucosal 5-HT concentrations and enteroendocrine cell numbers in subjects with d-IBS, as well as similar studies to the present one conducted in subjects with constipation predominant IBS and assessing the transient relationship between symptoms and the 5-HT system need to be performed.
Acknowledgments
This study was kindly supported by a grant from Glaxo Wellcome R&D, UK. We would like to thank EJ Fricker, LA Jacques, JG Mills, RSB Ehsanullah, and R Williamson, GlaxoSmithKline R&D, UK, for their help and support throughout this study.
Abbreviations
5-HT, 5-hydroxytryptamine
5-HIAA 5-hydroxyindole acetic acid
IBS, irritable bowel syndrome
d-IBS, diarrhoea predominant IBS
CV, coefficient of variation
AUC, area under the curve
REFERENCES
- 1.Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defaecation in the irritable bowel syndrome (IBS). Patients’ description of diarrhoea, constipation and symptom variation during a prospective 6 week study. Eur J Gastroenterol Hepatol 1998;10:415–21. [DOI] [PubMed] [Google Scholar]
- 2.Simren M, Mansson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001;63:108–15. [DOI] [PubMed] [Google Scholar]
- 3.Narducci F, Bassotti G, Granata MT, et al. Colonic motility and gastric emptying in patients with irritable bowel syndrome. Dig Dis Sci 1986;31:241–6. [DOI] [PubMed] [Google Scholar]
- 4.Rogers J, Henry MM, Misiewicz JJ. Increased segmental activity and intraluminal pressure in the sigmoid colon of patients with the irritable bowel syndrome. Gut 1989;30:634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan MA, Cohen S, Snape WJ. Colonic myoelectrical activity in irritable bowel syndrome. N Engl J Med 1987;298:878–83. [DOI] [PubMed] [Google Scholar]
- 6.Connell AM, Avery-Jones F, Rowlands EN. Motility of the pelvic colon. IV. Abdominal pain associated with colonic hypermotility after meals. Gut 1965;6:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans PR, Kellow JE. Physiological modulation of jejunal sensitivity in health and in irritable bowel syndrome. Am J Gastroenterol 1998;93:2191–6. [DOI] [PubMed] [Google Scholar]
- 8.Accarino AM, Azpiroz F, Malagelada J-R. Modification of small bowel mechanosensitivity by intestinal fat. Gut 2001;48:690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiller RC. Effects of serotonin on intestinal secretion and motility. Curr Opin Gastroenterol 2001;17:99–103. [DOI] [PubMed] [Google Scholar]
- 10.Kim D-Y, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol 2000;95:2698–709. [DOI] [PubMed] [Google Scholar]
- 11.Erspamer V, Testini A. Observations on the release and turnover rate of 5-hydroxytryptamine in the gastrointestinal tract. J Pharm Pharmacol 1959;11:618–23. [DOI] [PubMed] [Google Scholar]
- 12.Da Prada M, Tranzer JP, Pletscher A. Storage of 5-hydroxytryptamine in human blood platelets. Experimentia 1972;28:1328–9. [DOI] [PubMed] [Google Scholar]
- 13.Tyce GM. Biochemistry of serotonin. In: Van Houtte PM, ed. Serotonin and the Cardiovascular System. New York: Raven, 1985:1–14.
- 14.Bearcroft CP, Perrett D, Farthing MJG. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut 1998;42:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitaker P, Houghton LA, Madira W, et al. Gender differences and effects of age on 5-HT turnover: implications for irritable bowel syndrome. Gastroenterology 2001;120 (suppl 1):3226. [Google Scholar]
- 16.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut 1999;45(suppl II):42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bethea CL, Pecins-Thompson M, Schutzer WE, et al. Ovarian steroids and serotonin neural function. Mol Neurobiol 1998;18:87–123.10065876 [Google Scholar]
- 18.Whitaker RP, Dursun SM, Davies T, et al. Measurement of platelet rich plasma 5-HT and platelet poor plasma 5-HIAA and 5-HT in normal adults. Ann Clin Biochem 1996;B537:341 [Google Scholar]
- 19.Dursun SM, Whitaker RP, Andrews H, et al. Effects of aging on plasma 5-HT turnover in humans. Hum Psychopharm 1997;12:365–7. [Google Scholar]
- 20.Dursun SM, Szemis A, Andrews H, et al. Effects of clozapine and typical antipsychotic drugs on plasma 5-HT turnover and impulsivity in patients with schizophrenia: a cross-sectional study. J Psychiatr Neurosci 2000;25:347–52. [PMC free article] [PubMed] [Google Scholar]
- 21.Beck O, Wallen NH, Broijersen A, et al. On the accurate determination of serotonin in human plasma. Biochem Biophys Res Commun 1993;196:260–6. [DOI] [PubMed] [Google Scholar]
- 22.Blum I, Vered Y, Graff Y, et al. The influence of meal composition on plasma serotonin and norepinephrine concentrations. Metabolism 1992;41:137–40. [DOI] [PubMed] [Google Scholar]
- 23.Warner RRP. Hyperserotoninemia in function gastrointestinal disease. Annl Int Med 1963;59:464–76. [DOI] [PubMed] [Google Scholar]
- 24.Shimizudani T. Changes in the blood-serotonin levels in cases of upper gastrointestinal tract disease. Tokyo Ika Diagaku 1973;31:713–33. [Google Scholar]
- 25.McKee DP, Quigley EMM. Intestinal motility in irritable bowel syndrome: Is IBS a motility disorder? Part 1. Definition of IBS and colonic motility. Dig Dis Sci 1993;38:1761–72. [DOI] [PubMed] [Google Scholar]
- 26.McKee DP, Quigley EMM. Intestinal motility in irritable bowel syndrome: Is IBS a motility disorder? Part 2. Motility of the small bowel, esophagus, stomach and gall-bladder. Dig Dis Sci 1993;38:1773–82. [DOI] [PubMed] [Google Scholar]
- 27.Spiller RC. Disturbances in large bowel motility. In: Houghton LA, Whorwell PJ, eds. Irritable bowel syndrome. Baillieres Best Pract Res Clin Gastroenterol 1999;13:397–413. [DOI] [PubMed] [Google Scholar]
- 28.Quigley EMM. Disturbances in small bowel motility. In: Houghton LA, Whorwell PJ, eds. Irritable bowel syndrome. Baillieres Best Pract Res Clin Gastroenterol 1999;13:385–95. [DOI] [PubMed] [Google Scholar]
- 29.Pata C, Erdal E, Derici E, et al. Serotonin transporter gene polymorphism in irritable bowel syndrome. Am J Gastroenterology 2002;97:1780–4. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant IBS. Gastroenterology 2002;123:425–32. [DOI] [PubMed] [Google Scholar]
- 31.Nathan DM, Singer DE, Hurxthal K, et al. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med 1984;310:341–6. [DOI] [PubMed] [Google Scholar]
- 32.Cann PA, Read NW, Brown C. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut 1983;24:405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford MJ, Miller PM, Eastwood J, et al. Life events, psychiatric illness and the irritable bowel syndrome. Gut 1987;28:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welgan P, Meshkinpour H, Beeler M. Effect of anger on colon motor myoelectric activity in irritable bowel syndrome. Gastroenterology 1988;94:1150–6. [DOI] [PubMed] [Google Scholar]
- 35.Anthony M, Hinterberger H, Lance JW. Plasma serotonin in migraine and stress. Arch Neurol 1967;16:544–52. [DOI] [PubMed] [Google Scholar]
- 36.Elsenbruch S, Orr WC. Diarrhea- and constipation-predominant irritable bowel syndrome patients differ in post-prandial autonomic and cortisol responses. Am J Gastroenterol 2001;96:460–6. [DOI] [PubMed] [Google Scholar]
- 37.Pettersson G. The neural control of the serotonin content in mammalian enterochromaffin cells. Acta Physiol Scand Suppl 1979;470:1–30. [PubMed] [Google Scholar]
- 38.Miwa J, Echizen H, Matsueda K, et al. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhoea-predominant patients and subjects with normal bowel habit. Digestion 2001;63:188–94. [DOI] [PubMed] [Google Scholar]
- 39.Bose M, Nickols C, Greenwald S, et al. 5-hydroxytryptamine (5-HT) levels in colonic mucosa in the irritable bowel syndrome (IBS): assessment by high performance liquid chromatography (HPLC). Gut 2001;48:A57. [Google Scholar]