Abstract
Background and aims: The pathogenesis of asymptomatic diverticular disease (ADD) and symptomatic uncomplicated diverticular disease (SUDD) has not been elucidated. The aim of our study was to assess whether altered visceral perception or abnormal compliance of the colorectal wall play a role in these clinical entities.
Methods: Ten ADD patients, 11 SUDD patients, and nine healthy controls were studied. Using a dual barostat device, sensations were scored and compliance curves obtained using stepwise intermittent isobaric distensions of the rectum and sigmoid, before and after a liquid meal. In addition, the colonic response to eating was assessed by monitoring the volumes of both barostat bags at operating pressure before and after the meal.
Results: In the rectum, perception was increased in the SUDD group compared with controls (p = 0.010) and the ADD group (p = 0.030). Rectal compliance curves were not different between the groups. In the sigmoid colon, perception in the pre- and postprandial periods was increased in SUDD compared with controls (p = 0.018) but not when compared with ADD. Sigmoid volume-pressure curves had comparable slopes (compliance) in all groups but were shifted downwards in SUDD compared with ADD in the preprandial period (p = 0.026). The colonic response to eating (decrease in intrabag volume) was similar in all three groups, both in the rectum and sigmoid.
Conclusion: Symptomatic but not asymptomatic uncomplicated diverticular disease is associated with heightened perception of distension, not only in the diverticula bearing sigmoid, but also in the unaffected rectum. This hyperperception is not due to altered wall compliance.
Keywords: diverticular disease, visceral perception, colonic wall compliance, tone, barostat, sigmoid colon, rectum
Diverticular disease is a highly prevalent disorder in Western countries, the incidence rising with increasing age; up to 30% in those aged more than 60 years. Probably 20–25% of cases go undetected while 10–25% of patients under observation develop clinical signs of complications.1–4 Three categories of diverticular disease can be distinguished: (1) asymptomatic diverticular disease (ADD) in which multiple diverticula are found at colonoscopy or barium enema, without related symptoms; (2) symptomatic uncomplicated diverticular disease (SUDD) in which diverticula and abdominal pain are present, with or without irregular bowel function. SUDD is also known as painful diverticular disease; (3) symptomatic complicated diverticular disease: diverticular disease in which haemorrhage, peridiverticulitis, abscess, perforation, fistula, or bowel obstruction has developed.5
The pathogenesis of diverticular disease is still uncertain but is thought to be multifactorial. Patients with diverticular disease consume significantly smaller quantities of dietary fibre than age matched controls and geographic regions with low fibre intake have higher prevalence rates of diverticular disease.6–8 Another factor thought to be involved in the pathogenesis of diverticular disease is increased phasic motility in the diverticula bearing part of the colon but studies on this subject have yielded conflicting results.9–14 A change in bowel wall structure is thought to be another component in the development of diverticular disease.15,16 Information on the role of colonic tone in diverticular disease is still lacking.17
Symptoms in SUDD can be indistinguishable from those reported by patients with the irritable bowel syndrome (IBS). However, there are no data that indicate that IBS is a precursor of diverticular disease.3,15,18 In IBS, increased visceral perception, with a decreased volume and pressure threshold for urge and/or pain, was found in the rectum as well as at other intestinal sites and this abnormality is thought to be a hallmark of IBS.19–22
Only one study has been performed that examined wall characteristics and perception in an unselected group of patients with diverticular disease. The techniques used in this 1969 study (including use of water filled latex balloons) are now considered to be obsolete.9 Nowadays, the barostat technique is considered to be the optimal tool to measure compliance and visceral perception, either by isobaric or isovolumetric distensions.22–24
The aim of our study was to assess sigmoid and rectal visceral perception and wall characteristics in patients with asymptomatic and symptomatic uncomplicated diverticular disease and in healthy controls.
METHODS
Study subjects
Eleven patients (five men and six women), mean age 56 years (range 43–68), with a clinical diagnosis of SUDD were recruited from the outpatient clinic of the Department of Gastroenterology at the University Medical Centre Utrecht. This diagnosis was based on left lower quadrant abdominal pain, the presence of more than four diverticula in the sigmoid colon, as diagnosed by barium enema or colonoscopy, and absence of inflammatory or bleeding complications of diverticula in the medical history. The selected patients had a relatively short history of abdominal symptoms (2 months–6 years). Most of the patients fulfilled the Rome I symptomatic criteria for IBS but two patients had had left lower abdominal pain for two and three months, respectively, and therefore did not meet the time limit of the Rome I criteria (>6 months). Four of the SUDD patients were constipated, as defined by the Thompson criteria for constipation.25
Ten patients (six men and four women), mean age 56 years (range 43–69), with a diagnosis of ADD were selected from the colonic polyp surveillance programme. They were selected on the basis of having more than four diverticula in the sigmoid colon in the absence of abdominal complaints or complications of these diverticula at present or in the past. None fulfilled the Rome I criteria for IBS and two were constipated, as defined by the Thompson criteria.25
Nine healthy controls (six men and three women), mean age 51 years (range 42–61), were recruited by advertisement and from our own files.
None of the subjects had signs of systemic or gastrointestinal disease or a medical history of major abdominal surgery. None used medications on a regular base. All participants were asked to stop all incidentally used laxatives and bulk agents one week before the start of the protocol. Written informed consent was obtained from each subject and the ethics committee of the University Medical Centre Utrecht approved the study protocol.
Barostat device
A computer driven volume displacement device (Distender Series II Dual Drive Barostat; G&J Electronics Inc., Willowdale, Ontario, Canada) was used to inflate two polyethylene bags: one in the sigmoid colon and one in the rectum. The barostat device contained two independently functioning cylinders acting as non-compliant bellows, each having a capacity of 1200 ml. Non-compliant tubes connected these reservoirs to polyethylene bags. The barostat maintained a constant and preselected pressure level in the bag by an electromechanical feedback mechanism, and continuously measured intrabag volume. In response to any change in pressure in the bag, the barostat injected or withdrew air to maintain the preselected pressure. Thus the recorded changes in volume over time reflected changes in colonic tone.
The barostat apparatus included a built-in computer system that could be programmed to automatically perform distensions with fixed time lag and bag pressure increments for both cylinders independently. At each pressure, the barostat automatically calculated corrected volumes according to Boyle’s law.
In this experiment we used the barostat to perform intermittent distensions, deflating the bag between each pressure driven distension step, at an air flow rate of 1.9 l/min.
Colonic assemblies
A double lumen non-compliant polyethylene tube (Dantec Medical, Skovlunde, Denmark) incorporating a polyethylene bag at 15 cm from the tip was used to perform distensions in the sigmoid colon. A similar polyethylene tube incorporating a polyethylene bag at its tip was used to perform distensions in the rectum. The channel for air injection and evacuation had an inner diameter of 6 mm in both catheters, allowing a maximum air flow of 35 ml/s. The second channel had its side hole in the barostat bag and this was used to measure the pressure in this bag. To each of the catheters a thin walled (40 μm thick) polyethylene cylindrical bag was attached. The maximum capacity of these bags was 800 ml, their maximum diameter was 10 cm (during table top inflation), and their length was 10 cm. Before each experiment, the bags, catheters, and barostat were checked for air leaks by submerging the bags under water while maintaining a constant pressure of 20 mm Hg.
Study protocol
At 8.00 am, participants were admitted to the clinical research centre after an overnight fast. The colon was cleaned using a 1.5 litre enema of polyethylene glycol and electrolytes (Klean-Prep; Norgine, Utrecht, the Netherlands). At 9.00 am, the sigmoid catheter incorporating the barostat bag was placed endoscopically. The tip of the catheter was attached to the colonoscope and introduced until the tip of the catheter reached the descending colon and the bag was located in the diverticular part of the sigmoid colon. The procedure was performed without sedation and with minimal insufflation of air. Then the second probe with the polyethylene bag at the tip was introduced into the rectum without endoscopic assistance. The position of the sigmoid barostat bag was verified by fluoroscopy.
After introduction of the probes, all subjects were in a 30° supine position during the entire recording session and they were asked not to make unnecessary movements.
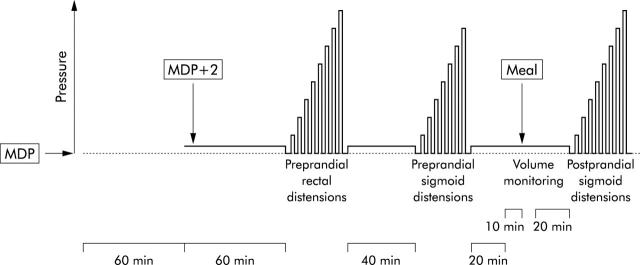
Figure 1 ▶ illustrates the study protocol. One hour after placement of the probes, the minimum distending pressure (MDP) was determined for both rectal and sigmoid bags by recording the lowest pressure at which respiratory excursions were regularly recorded as changes in barostat volumes. After another hour of baseline recording, with both bags at MDP+2 mm Hg, a series of eight stepwise intermittent isobaric distensions (maintained for two minutes) were performed with 4 mm Hg increments, deflating the rectal barostat balloon to MDP between two distensions over two minutes. The maximal pressure reached was 32 mm Hg above MDP (distension step 8) or the pressure at which the subject perceived the maximal tolerable pain. After this rectal series and a 40 minute baseline period at operating pressures, the same series as described above was performed in the sigmoid colon, the maximal pressure reached being 28 mm Hg above MDP (distension step 7) or the pressure at which the subject perceived the maximal tolerable pain.
Figure 1.
Schematic representation of the study protocol. MDP, minimum distending pressure.
After 20 minutes of accommodation, with both bags at MDP+2 mm Hg, a 10 minute preprandial recording period was followed by ingestion of a 500 ml, 600 kcal (35% fat, 49% glucose, 16% protein) liquid meal (Nutridrink; Nutricia, Zoetermeer, the Netherlands) that was consumed in five minutes, followed by a 20 minute postprandial recording period. Subsequently, the same sigmoid distension procedures were carried out as described for the preprandial period.
During both rectal and sigmoid distension, the intensity of sensation to each distension step was scored. Prompted by a red light one minute after the start of each distension, subjects were asked to rate their sensation by pushing one button of an array of 7. Button 1 indicated “no sensation” and button 7 “maximal tolerable pain”.
During distension of the rectum or sigmoid bag, the pressure in the other bag (sigmoid and rectal, respectively) was maintained at operating pressures. Subjects were instructed that they had the option to deflate the bags instantaneously at any time if they experienced significant discomfort by pressing a button on their electronic control panel. Subjects had no visual or auditory clues to anticipate the type or course of distensions.
After fluoroscopic control of the catheter and sigmoid bag position, the experiment was finished and the probes removed. Duration of the experiment from probe placement until their removal was approximately five hours.
Parameters investigated
Perception score
Mean sensation score was assessed for every distension step.
Compliance
Volumes measured one minute after the onset of each of the distensions were used to construct the pressure-volume curves. The dV/dP relationship was analysed by calculating the slope of the pressure-volume curve by means of linear regression analysis resulting in a compliance coefficient.
Barostat volume tracings
Mean volume at “operating pressure” in both the rectal and sigmoid barostat was for three 10 minutes periods; one before the meal and two 10 minute periods after the meal, using a computer program (Protocol Plus data scanner; G&J Electronics Inc.).
Statistical analysis
Data are expressed as mean (SEM). Differences in compliance curves and perception intensity curves between groups and between the pre- and postprandial states within groups were analysed with a general linear model (GLM) for repeated measures. Paired t tests for within-patient comparisons and t tests for group comparisons were used to evaluate differences between compliance coefficients.
For analysis of differences in barostat volumes during the total periprandial period between groups, a GLM for repeated measures was used. Paired t tests for single patient comparisons and independent t tests for group comparisons were used to evaluate differences between volumes in the separate periprandial periods. All analyses were conducted using the SPPS 7.0 statistical package.
RESULTS
All subjects completed the experiment. None of the subjects used the emergency button on the control panel to deflate the balloon because of unbearable discomfort. None of the barostat bags was dislocated during the experiment, as checked by fluoroscopy.
Perception score
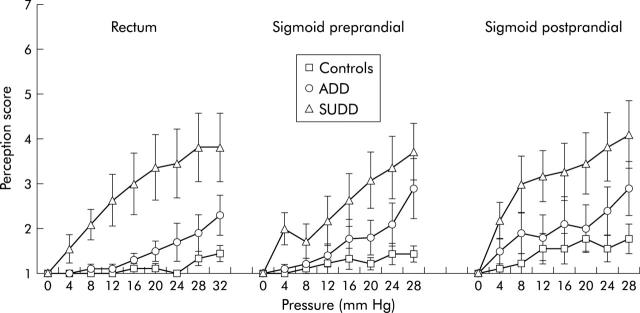
In the rectum, perception scores in the distension series were significantly higher in the SUDD group than in controls (p = 0.010) or the ADD group (p = 0.030). No difference in perception scores was found between ADD and controls (fig 2 ▶).
Figure 2.
Perception (score 1 = no sensation, score 7 = maximal tolerable pain) on stepwise isobaric distensions of the rectum and sigmoid colon during the preprandial period and in the sigmoid colon during the postprandial period in healthy controls, asymptomatic diverticular disease (ADD) patients, and symptomatic uncomplicated diverticular disease (SUDD) patients. In the rectum, the SUDD group had increased perception scores compared with the control group (p = 0.010) and the ADD group (p = 0.030). In the sigmoid colon, in the pre- and postprandial periods, the SUDD group had increased perception scores compared with the control group (p = 0.018).
In the sigmoid colon, preprandial perception scores in the distension series were significantly higher in the SUDD group than in controls (p = 0.018) but not compared with the ADD group.
Postprandially, comparable results were found, with perception scores being significantly higher in the SUDD group than in controls (p = 0.018) but were not significantly different from the ADD group.
There were no significant differences in perception scores between ADD and controls, either pre- or postprandially (fig 2 ▶).
Compliance
Operating pressures for the barostat bags in the sigmoid colon and rectum were not significantly different between controls (15 (1.3) mm Hg and 18 (1.2) mm Hg, respectively), ADD (17 (1.1) mm Hg and 19 (0.9) mm Hg, respectively), and SUDD patients (17 (1.2) mm Hg and 20 (0.7) mm Hg, respectively).
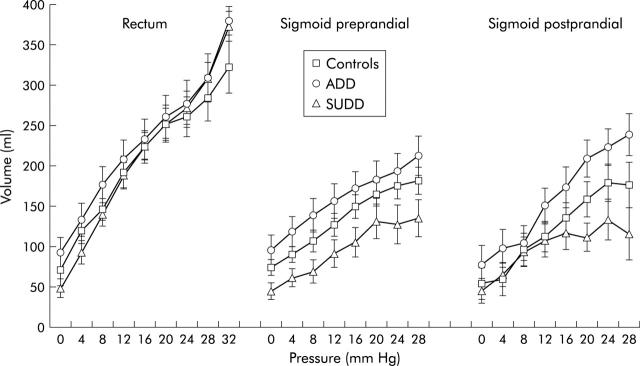
There were no differences between the three groups in the slope of the volume-pressure curves (dV/dP) in the rectum or sigmoid colon (fig 3 ▶, table 1 ▶). However, in the sigmoid colon, preprandial volumes in SUDD patients were significantly lower than in ADD patients (p = 0.026) due to a lower volume at MDP in the SUDD group. In the postprandial period a trend towards the same phenomenon was found (p = 0.079).
Figure 3.
Volume-pressure curves in the rectum and sigmoid colon during the preprandial period and in the sigmoid colon in the postprandial period on isobaric distensions in healthy controls, asymptomatic diverticular disease (ADD) patients, and symptomatic uncomplicated diverticular disease (SUDD) patients. Preprandially, the SUDD curve was shifted downwards compared with the ADD curve (*p = 0.026).
Table 1.
Compliance (ml/mm Hg) in the rectum and sigmoid colon
| Controls | ADD | SUDD | |
| Rectum | 7.5 (0.1) | 7.1 (0.7) | 9.2 (0.9) |
| Sigmoid preprandial | 4.1 (0.5) | 3.7 (0.5) | 4.1 (1.2) |
| Sigmoid postprandial | 4.7 (0.7) | 4.6 (0.9) | 3.9 (0.9) |
Data are mean (SEM).
ADD, asymptomatic diverticular disease; SUDD, symptomatic uncomplicated diverticular disease.
Ingestion of the meal had no significant effect on compliance (fig 3 ▶, table 1 ▶).
Periprandial volume variations in the rectum and sigmoid colon
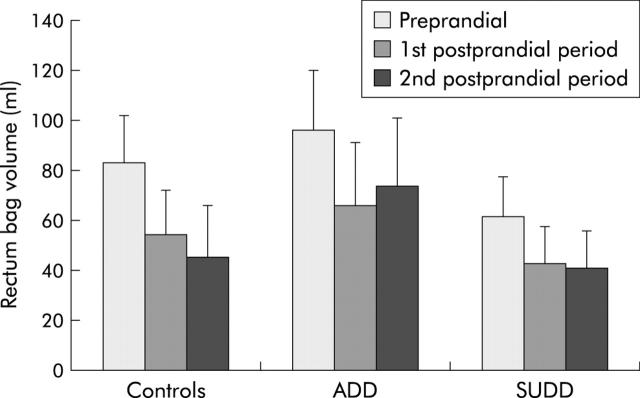
In the rectum, no significant differences in intrabag volume changes at operating pressure were found between the groups, for the total periprandial period or for the magnitude of reduction of rectal volume after the meal. In all three groups, postprandial rectal volume was significantly lower than preprandial rectal volume (controls p = 0.006; ADD p = 0.016; SUDD p = 0.003), representing a physiological postprandial increase in tone (fig 4 ▶).
Figure 4.
Barostat volumes in the rectum before the meal (Preprandial), and in the first and second 10 minute postprandial periods. There were no significant differences between the groups (healthy controls, asymptomatic diverticular disease (ADD) patients, and symptomatic uncomplicated diverticular disease (SUDD) patients). In all three groups, rectal volume decreased significantly after the meal (controls p = 0.006; ADD p = 0.016; SUDD p = 0.003).
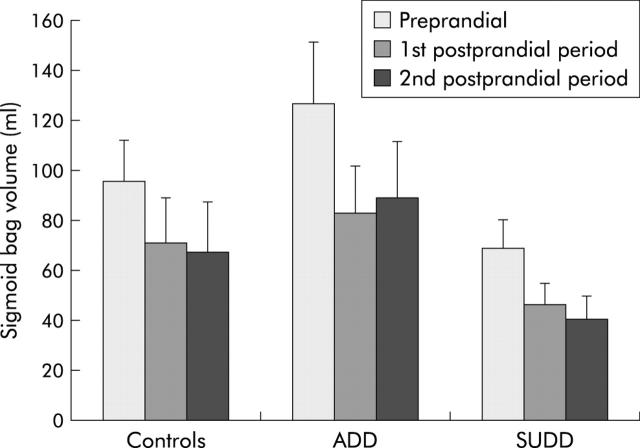
In the sigmoid colon, barostat volumes in the total periprandial period did not differ significantly in either of the patient groups compared with controls. All groups had a prompt and highly significant decrease in sigmoid barostat volumes after ingestion of the meal (controls p = 0.001; ADD p = 0.001; SUDD p = 0.004). The volume reached in the first 10 minute postprandial period was maintained in the second 10 minute postprandial period. The magnitude of the volume reduction was not significantly different between the groups (fig 5 ▶).
Figure 5.
Barostat volumes in the sigmoid colon before the meal (Preprandial), and in the first and second 10 minute postprandial periods. Differences between the groups failed to reach statistical significance. In all three groups (healthy controls, asymptomatic diverticular disease (ADD) patients, and symptomatic uncomplicated diverticular disease (SUDD) patients) sigmoid volumes decreased significantly after the meal (controls p = 0.001; ADD p = 0.001; SUDD p = 0.004).
DISCUSSION
In this study, we investigated two groups of patients with uncomplicated diverticulosis of the colon: one with asymptomatic diverticular disease (ADD) and one with symptomatic uncomplicated diverticular disease (SUDD), also called painful uncomplicated diverticular disease. We wished to examine whether visceral perception of the distension stimulus is different in these clinically distinct entities and, if so, whether the differences could be explained by changes in compliance of the rectosigmoid.
In the sigmoid colon, perception in the pre- and postprandial periods was increased in SUDD patients compared with controls. Rather unexpectedly, we also observed increased perception in the rectum of SUDD compared with ADD and controls. As discussed below, this increase in pain perception in the SUDD group was not due to a change in rectal wall characteristics. Thus in SUDD, increased perception appears to be present not only in the diverticula bearing sigmoid colon but also in the unaffected rectum. This observation gives rise to the suggestion that patients with SUDD are in fact IBS patients who also happen to have diverticulosis. Increased visceroperception in the rectum as well as in other parts of the alimentary canal is a well known feature of IBS.19,21 It can be argued that some clinical observations suggest that IBS and SUDD are two distinct conditions without progression of one to the other. IBS patients often have a long history of abdominal complaints, starting at a young adult age, whereas in many patients with symptomatic uncomplicated diverticular disease the onset of abdominal pain is shortly before the discovery of their diverticula.3,15,18 However, these observations do not exclude the possibility that IBS patients with late symptom onset whose pre-existent diverticulosis is incidentally discovered during diagnostic workup, are erroneously labelled as SUDD patients.
In our patients with uncomplicated diverticular disease, bowel wall compliance was normal, not only in the rectum but also in the sigmoid (that is, resistance to distension was similar in ADD, SUDD, and health). This was also an unexpected finding as a change in bowel wall structure is thought to be one of the components for the development of diverticular disease. In diverticular disease, the amount of elastin in taeniae coli is increased, causing shortening of taeniae and “upbunching” of muscle, mesentery, and mucosa. The lumen narrows, the muscle layer seems thicker, and the gut is shortened.15,16 One would expect that these changes could lead to decreased compliance of the gut wall. However, in our study the SUDD group had significantly lower sigmoid volumes on every pressure step compared with the ADD group, without a change in wall compliance. This indicates that basal sigmoid tone in SUDD is increased compared with ADD.
Whereas no alteration in compliance was observed in our study, the only other distension study in diverticular disease showed decreased resistance to stretch of the sigmoid wall.9 Postmortem distension studies by the same investigators yielded the same results.26 It is now accepted that the water filled latex balloons that were used in the studies are far from ideal for studying colonic wall characteristics. Firstly, a latex balloon has a compliance of its own that has to be corrected for. Secondly, at certain critical pressures, a latex balloon looses its elastic properties and becomes plastic, resulting in a balloon that can accommodate large volumes with little increase in pressure.24,27 Therefore, the results of our study cannot be compared with those obtained with a latex balloon.
A dual barostat device enabled us to measure simultaneously rectal and sigmoid volume over a 30 minute periprandial observation period. When intraluminal pressure is kept constant, changes in colonic tone caused by contraction or relaxation of the colonic smooth muscle lead to sustained changes in luminal cross sectional area and circumference. As colonic surface area cannot easily be measured directly in vivo, it is assumed that the colon and rectum roughly behave like cylindrical structures, implying that variations in volume, as measured by the barostat, reflect fluctuations in colonic diameter, and these are thought to reflect variations in tone of the bowel wall.17,28 In our study, rectal volume at MDP+2 mmHg and the postprandial decrease in rectal volume were not significantly different between groups. Sigmoid colonic volumes tended to be lower in the SUDD group than in the ADD group but there was no statistically significant difference. The three groups had comparable postprandial decreases in sigmoid volume. These findings indicate no major differences in colonic tone between symptomatic and asymptomatic subjects with diverticulosis. The findings indicate that the normal postprandial response to feeding is preserved in diverticular disease.
In summary, this study has shown that patients with SUDD show heightened visceral perception of rectosigmoid distension stimuli which is not found in ADD. This hyperperception is not limited to the diverticula bearing sigmoid colon and is not due to altered compliance of the gut wall. These findings indicate a generalised hyperperception of intestinal stimuli in symptomatic diverticulosis which resembles IBS. A study on perception and colonic wall characteristics in SUDD patients compared with age matched patients with a long history of IBS but without diverticula may resolve the remaining questions.
Acknowledgments
Dr M Samsom is a fellow of the Royal Netherlands Academy of Arts and Sciences.
Abbreviations
ADD, asymptomatic diverticular disease
SUDD, symptomatic uncomplicated diverticular disease
IBS, irritable bowel syndrome
MDP, minimum distending pressure
GLM, general linear model for repeated measures
REFERENCES
- 1.Connell AM. Pathogenesis of diverticular disease of the colon. Adv Intern Med 1977;22:377–95. [PubMed] [Google Scholar]
- 2.Painter NS, Burkitt DP. Diverticular disease of the colon, a 20th century problem. Clin Gastroenterol 1975;4:2–21. [PubMed] [Google Scholar]
- 3.Almy TP, Howell DA. Diverticular disease of the colon. New Engl J Med 1980;302:324–31. [DOI] [PubMed] [Google Scholar]
- 4.Whiteway J, Morson BC. Pathology of the ageing—diverticular disease. Clin Gastroenterol 1985;14:829–46. [PubMed] [Google Scholar]
- 5.Cheskin LJ, Lamport RD. Diverticular disease. Epidemiology and pharmacological treatment. Drugs Aging 1995;6:55–63. [DOI] [PubMed] [Google Scholar]
- 6.Brodribb AJM, Humphreys DM. Diverticular disease: three studies, part I—Relation to other disorders and fibre intake. BMJ 1976;1:424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gear JSS, Ware A, Fursdon P, et al. Symptomless diverticular disease and intake of dietary fibre. Lancet 1979;10:511–14. [DOI] [PubMed] [Google Scholar]
- 8.Burkitt DP, Wolker A, Painter NS. Effects of dietary fiber on stools and the transit time and its role in the causation of disease. Lancet 1972;2:1408–12. [DOI] [PubMed] [Google Scholar]
- 9.Parks TG, Connell AM. Motility studies in diverticular disease of the colon. Gut 1969;10:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Painter NS, Truelove SC. The intraluminal pressure patterns in diverticulosis of the colon. Gut 1964;5:201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Painter NS, Truelove SC, Ardran GM, et al. Segmentation and the localisation of intraluminal pressures in the human colon. Gastroenterology 1965;49:169–77. [PubMed] [Google Scholar]
- 12.Weinreich J, Andersen D. Intraluminal pressure in the sigmoid colon. II Patients with sigmoid diverticula and related conditions. Scand J Gastroenterol 1976;11:581–6. [PubMed] [Google Scholar]
- 13.Trotman IF, Misiewicz JJ. Sigmoid motility in diverticular disease and the irritable bowel syndrome. Gut 1988;29:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassotti G, Battaglia E, Spinozzi F, et al. Twenty-four hour recordings of colonic motility in patients with diverticular disease. Dis Colon Rectum 2001;44:1814–19. [DOI] [PubMed] [Google Scholar]
- 15.Smith AN. Colonic muscle in diverticular disease. Clin Gastroenterol 1986;15:917–35. [PubMed] [Google Scholar]
- 16.Watters DAK, Smith AN. Strength of the colon wall in diverticular disease. Br J Surg 1990;77:257–9. [DOI] [PubMed] [Google Scholar]
- 17.Jouet P, Coffin B, Lemann M, et al. Tonic and phasic motor activity in the proximal and distal colon of healthy humans. Am J Physiol 1998;274:G459–64. [DOI] [PubMed] [Google Scholar]
- 18.Thompson WG, Patel DG. Clinical picture of diverticular disease of the colon. Clin Gastroenterol 1986;15:903–6. [PubMed] [Google Scholar]
- 19.Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distension in irritable bowel syndrome. Gastroenterology 1990;98:1187–92. [DOI] [PubMed] [Google Scholar]
- 20.Trimble KC, Farouk R, Pryde A, et al. Heightened visceral sensation in functional gastrointestinal disease is not site-specific. Evidence for a generalized disorder of gut sensitivity. Dig Dis Sci 1995;40:1607–13. [DOI] [PubMed] [Google Scholar]
- 21.Mertz H, Naliboff BD, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995;109:40–52. [DOI] [PubMed] [Google Scholar]
- 22.Bradette M, Delvaux M, Stoumont G, et al. Evaluation of colonic sensory thresholds in IBS patients using a barostat. Dig Dis Sci 1994;39:449–57. [DOI] [PubMed] [Google Scholar]
- 23.Lembo T, Munakata J, Naliboff B, et al. Sigmoid afferent mechanisms in patients with irritable bowel syndrome. Dig Dis Sci 1997;42:1112–20. [DOI] [PubMed] [Google Scholar]
- 24.Toma TP, Zighelboim J, Phillips SP, et al. Methods for studying intestinal sensitivity and compliance: in vitro studies of balloons and a barostat. Neurogastroenterol Mot 1996;8:19–28. [DOI] [PubMed] [Google Scholar]
- 25.Thompson WG, Heaton KW. Functional bowel disease in apparently healthy people. Gastroenterology 1979;79:283–7. [PubMed] [Google Scholar]
- 26.Parks TG. Rectal and colonic tone studies after resection of the sigmoid for diverticular disease. Gut 1970;4:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akervall S, Fasth S, Nordgen S, et al. Rectal reservoir and sensory function studied by graded isobaric distensions in normal man. Gut 1989;30:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregersen H, Kassab G. Biomechanics of the gastrointestinal tract. Neurogastroenterol Mot 1996;8:277–97. [DOI] [PubMed] [Google Scholar]