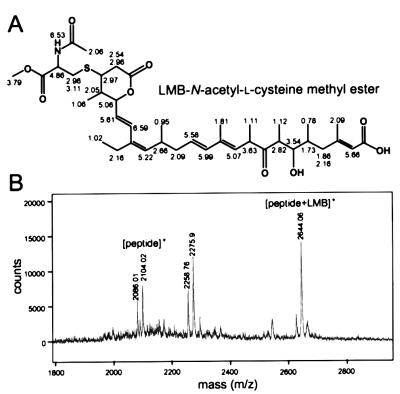
Figure 2.
Covalent binding of leptomycin B to N-acetyl-l-cysteine methyl ester and a peptide containing a cysteine. (A) Chemical structure of an LMB complex with N-acetyl-l-cysteine methyl ester and the chemical shifts of 1H NMR. An adduct generated in the mixture containing LMB and N-acetyl-l-cysteine methyl ester was purified by HPLC, and the structure was determined by 1H NMR and correlated spectroscopy. The chemical shifts are presented with the structure. (B) An addition reaction with a peptide containing a cysteine residue. An 18-aa peptide corresponding to hCRM1 residues 513–530 that contains Cys-528 was incubated with LMB for 24 h at 37°C and analyzed by matrix-assisted laser desorption ionization–TOF MS. A peak of 2,275.9 Da may be a product of the reaction between the peptide and α-cyano-4-hydroxycinnamic acid (αCHCA) used as the matrix. Calculated molecular mass (Da) is 540 for LMB, 2,104.54 for the peptide, and 189.04 for αCHCA.