Figure 1.
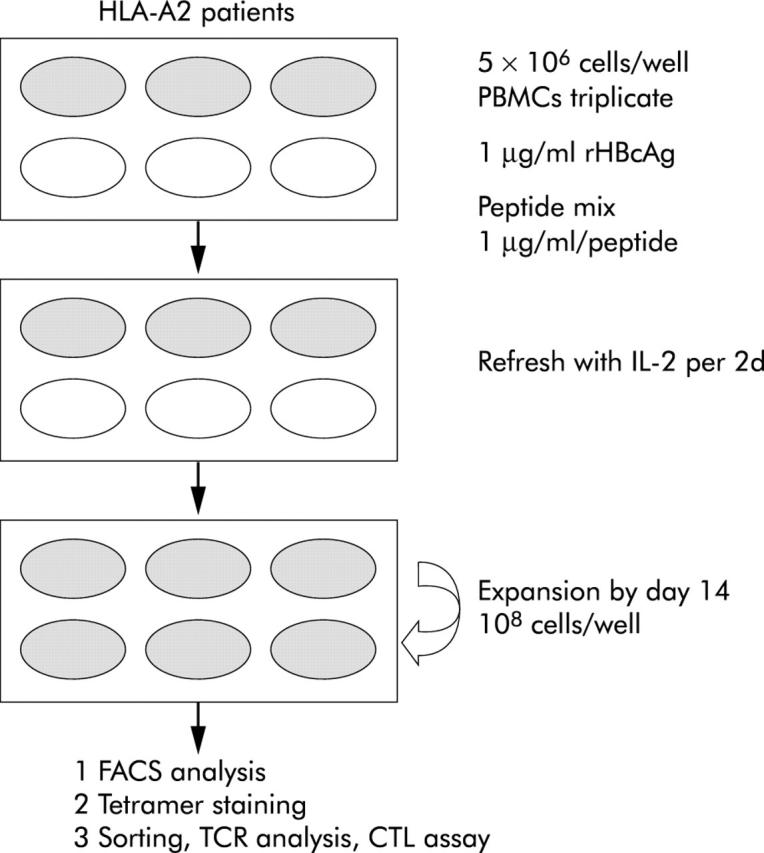
Protocol of the modified CTL response index of the peptide (CRI-p) culture method. On the first day of blood sampling, 5×106 peripheral blood mononuclear cells (PBMCs) in complete medium in triplicate cultures in a six well flat bottomed plate (Nunc, Roskilde, Denmark) with a mixture of the panel of peptides to be tested (each 1 μg/ml), 1 μg/ml recombinant hepatitis B core antigen (rHBcAg)32, and 20 U/ml recombinant human interleukin 2 (rhIL-2) were incubated at 37°C in a humidified 5% CO2 incubator. On days 3, 5, 7, 9,11, and 13, the culture medium was refreshed with 20 U/ml of rhIL-2. Usually, the total number of cells could proliferate up to 3∼5×107 cells on day 13, and sometimes to 108 cells by day 14. Cells harvested on days 14–15 were used for cell lytic assays and phenotypic study, with surface staining of MHC-A2-peptide tetrameric complexes. CTL, cytotoxic T lymphocyte; TCR, T cell receptor.
