Abstract
Background: We previously described hepatitis reactivation in two carriers of the hepatitis C virus (HCV) genotype 2c.
Aim: To assess the relationship between HCV genotypes and risk of hepatitis reactivation, we studied the course of aminotransferases in patients infected with the two relevant genotypes in Italy.
Patients: A cohort of 100 patients with genotype 2c chronic hepatitis and 106 with genotype 1b were subjected to surveillance.
Methods: Hepatitis reactivation was defined as an alanine aminotransferase (ALT) value ⩾400 IU/l or a maximum/minimum ALT ratio value of ⩾8.
Results: Over a period of 71 (24–144) months, one or more flares of ALT (201–2200 IU/l, 6–90 months’ duration) occurred in 31 patients with genotype 2c and in eight patients with genotype 1b (rates of flares: 55.6 per 1000 person years for genotype 2c v 15.0 for genotype 1b; p = 0.001). On repeat biopsy, hepatic fibrosis increased by more than 2 points in 10/16 patients examined either during or after an ALT flare compared with 7/36 flare free patients (63% v 19%; p = 0.003). Hepatitis flares were significantly associated with genotype 2c (odds ratio 6.48 (95% confidence interval 2.57–16.35)) but not with sex, age, modality or duration of infection, baseline ALT values or histological severity of hepatitis, hepatitis other than HCV, or reinfection.
Conclusions: Genotype 2c carriers are at high risk of hepatitis reactivation, suggesting that virus genetic heterogeneity is important in the natural history of HCV, questioning the linearity of hepatic fibrosis progression during hepatitis C.
Keywords: hepatitis C virus, virus genotype, chronic hepatitis, hepatitis reactivation, aminotransferases
Hepatitis C virus (HCV) has a remarkably heterogeneous genetic organisation and clinical expression.1,2 In the face of the many chronically infected patients who developed an indolent slowly progressive hepatitis characterised by persistently normal or near normal values of serum alanine aminotransferase (ALT) and mild histological lesions on biopsy,3–6 the majority of patients have a more active disease that may ultimately progress to cirrhosis and hepatocellular carcinoma.7–9 Older age at the time of infection, male sex, alcohol abuse, and immunosuppression are relevant host factors thought to increase the risk of progressive liver disease in HCV patients.10–12 In contrast, there is little evidence that the risk of progression of hepatitis C is affected significantly by virological factors, including virus genotype.8,13–16 The only clear cut correlation that has emerged between virus genotype and outcome of hepatitis C is the better response to interferon therapy of genotype 2 or 3 patients compared with genotype 1 or 4 patients.17
Recently, we described two carriers of genotype 2 in whom serum ALT suddenly flared up after eight and 15 years of indolent infection, respectively, and the original mild hepatitis on biopsy progressed to severe liver fibrosis.18 These observations led us to speculate on whether hepatitis C reactivation could be closely related to genotype 2 infection and whether the prognosis of hepatitis C could be accurately predicted by ALT assessment. A prospective study to assess the course of hepatitis C in relation to virus genotype appears unfeasible at present due to the highly effective anti-HCV treatments available that have reduced the number of patients left untreated. Re-analysis of a cohort of patients who in the 1990s were left untreated offered us an opportunity to evaluate the frequency of hepatitis C reactivation and its relationship to virus genotype. We report here the rates and outcome of ALT flares in a cohort of patients infected with the two most prevalent HCV genotypes in Italy—that is, genotypes 1b and 2a/c—who were followed untreated for a mean period of 71 months.
MATERIAL AND METHODS
Study design
The cohort included all patients with genotypes 2a/c who had been consecutively enrolled in our centre between 1990 and 1993 and who had remained under observation for at least two years without receiving interferon (IFN) therapy. For comparison, we identified a similar number of patients from hospital files sorted alphabetically infected by genotype 1b who had remained untreated for a similar length of time. We excluded patients infected by other genotypes as they could not be recruited in sufficient number for comparison with genotypes 2c and 1b. Genotypes 1a, 3a, and 4 together accounted for less than 20% of patients attending our centre in the 1990s.19 Patients were also excluded if they had serum hepatitis B surface antigens (HBsAg), daily alcohol intake >60 g for men and >40 g for women, serum antibodies to human immunodeficiency virus, or autoimmune liver disease.
Clinical and laboratory examinations were carried out in patients every six months prior to an ALT flare. Patients found to have an ALT flare were examined at closer intervals. During the observation period, all patients were offered a diagnostic liver biopsy. IFN therapy was offered to patients aged 18–65 years with either persistently or intermittently high ALT values and a liver biopsy showing moderate or severe chronic viral hepatitis (grading ⩾7; staging ⩾2).20
Definition of ALT flare
An ALT flare was arbitrarily defined as an ALT value ⩾400 IU/l or a maximum/minimum ALT ratio value of ⩾8. The end of a flare was defined as a fall in ALT below twice the mean ALT value preceding the flare. To exclude causes other than HCV for the ALT flare, patients were tested for hepatitis A virus (HAV), hepatitis B virus (HBV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), toxoplasma virus and herpes viruses (HVS1 and HVS2), and tissue autoantibodies, and were rigorously questioned on consumption of alcohol, drugs, or herbal remedies. Reinfection was excluded by thorough questioning of patients for sexual or parenteral exposure and testing for hepatitis viruses. Retrospectively, all flaring patients were also retested for HCV genotype.
Measurements
Serum ALT and aspartate aminotransferase (AST) activities were measured by an automated method at 37°C (normal value ⩽37 IU/l). Commercially available enzyme immunoassays were used to determine serum HBsAg, antibodies to hepatitis B core antigen (anti-HBc), antibodies to HAV, antibodies to CMV, antibodies to EBV, antibodies to HSV1 and HSV2, and antibodies to toxoplasma. Antibodies to nuclear, smooth muscle, mitochondrial, and liver and kidney microsomal antigens were assayed on rat liver and kidney cryostat sections by immunofluorescence. Antinuclear antibodies were confirmed on Hep 2 cells. Serum HCV-RNA was assessed by nested reverse transcription-polymerase chain reaction (RT-PCR) using specific primers from the 5′ non-coding region. The sensitivity of the assay was 50 HCV-RNA copies/ml. HCV was genotyped by a hybridisation assay (Inno-LIPA; Innogenetics, Ghent, Belgium). HCV genotypes 2a/c were subtyped using type specific primers of the core region.21 When nested type specific PCR was unable to differentiate subtype 2a from 2c, direct sequencing of the core region (nt 160–356) was performed. This fragment had previously proved to be valuable for identification of subtype 2c.22 The sequences obtained were aligned with the consensus sequences of subtypes 2c and 2a. Serum HCV-RNA was quantitatively measured by a branched DNA signal amplification assay (Versant HCV-RNA 3.0 Assay bDNA; Bayer, Emeryville, California, USA) and expressed as IU/l.
All patients were offered a diagnostic liver biopsy with a Tru Cut needle (gauge 16; Travenol Hyland, TSK Laboratories, Tokyo, Japan). A follow up biopsy was offered to all patients who were left untreated to assess disease progression and treatment options. The severity of hepatic inflammation was evaluated by the Ishak score in separate reports for grading and staging. The maximum score for grading was 18, ranging from 0 to 4 for piecemeal necrosis, focal necrosis, and portal inflammation, and from 0 to 6 for confluent necrosis. The maximum score for staging was 6, with 0 representing no fibrosis and 6 cirrhosis20 In paired liver biopsy samples, the rate of fibrosis progression was calculated as the difference between the scores on two consecutive biopsies divided by the time in years between the two biopsies (fibrosis units per year).10
Statistical analysis
The odds ratio (OR) and corresponding 95% confidence interval (CI) of having an ALT flare were derived from a multiple logistic regression model23 which included terms for age, sex, duration of HCV infection (<5/5–9/⩾10 years), liver histology staging (<3/3–4/⩾5), modality of infection (community acquired/parenteral), basal value of ALT (mean of the first three observations), anti-HBc (positive/negative), genotype, and number of observations.
Univariate data comparisons among groups were performed with χ2 tests for proportions and Student’s t tests for means.
RESULTS
Patients
The index cases were 100 patients with genotype 2a/c chronic hepatitis C, originating from various regions in Italy (table 1 ▶): 106 genotype 1b patients were enrolled for comparison from hospital files sorted alphabetically. Patients represented 65% of all genotype 1b patients registered in our centre in the early 1990s. By sequence analysis, 99 2a/c specimens were found to be genotype 2c. A diagnostic liver biopsy was available for 85 patients with genotype 2c and for 102 patients with genotype 1b. In fact, 15 patients with persistently normal ALT values refused liver biopsy whereas four patients with advanced cirrhosis were not eligible for the procedure.
Table 1.
Characteristics of the 206 patients enrolled in the cohort study
| HCV 1b | HCV 2c | |
| No of patients (males) | 106 (60) | 100 (60) |
| Mean (SD) age (y) | 48 (10) | 49 (9) |
| Mean (SD) disease duration (months) | 91 (85) | 94 (100) |
| Modality of infection | 24 (23%) | 24 (24%) |
| Transfusion | 23 (22%) | 23 (23%) |
| Occupational/sex | 1 (1%) | 1 (1%) |
| Unknown | 82 (77%) | 76 (76%) |
| ALT (IU/l)* | 114 (18–308) | 137 (10–514) |
| Anti-HBc | ||
| Negative | 88 (83%) | 82 (82%) |
| Positive | 18 (17%) | 18 (18%) |
| Bilirubin (mg/dl) | 0.7 (0.4–13) | 0.7 (0.6–14) |
| Histological grading | ||
| 3–7 | 58 (55%) | 45 (45%) |
| 8–10 | 35 (33%) | 28 (28%) |
| 11–15 | 9 (8%) | 12 (12%) |
| Histological staging | ||
| 0–2 | 54 (51%) | 46 (46%) |
| 3/4 | 20 (19%) | 17 (17%) |
| 5/6 | 28 (26%) | 22 (22%) |
*Median (range).
ALT, alanine aminotransferase; HCV, hepatitis C virus.
The two genotype groups of patients were similar in age, sex distribution, modality and duration of infection, baseline ALT values, and degree of histological inflammation at biopsy. Patients were followed for a mean period of 71 months (range 24–144) without receiving any treatment. Sixty four patients with genotype 2c and 58 with genotype 1b were ultimately treated with IFN alone or in combination with ribavirin after a mean treatment free period of 44 months (range 24–138). The average treatment free interval was 49 months for patients with genotype 2c and 38 months for those with genotype 1b. Eighty four patients were left untreated for an average period of 110 months (range 54–144) because they were more than 65 years old (n = 4), had ALT values <1.5 times the upper limit of normal (n = 30), a clinical diagnosis of cirrhosis (n = 6), trivial lesions at biopsy (n = 14), psychological depression (n = 11), high levels of tissue autoantibodies (n = 2), comorbidities (n = 7), or refused to be treated (n = 10).
ALT monitoring
During 71 months of follow up, three patterns of serum ALT abnormalities were observed: (i) persistently normal or near normal ALT values occurred in 19 patients (9%); (ii) 148 (78%) patients had either persistently or intermittently high ALT values; and (iii) 39 patients with either ALT pattern had an ALT flare (19%; 31 with genotype 2c and eight with genotype 1b) (fig 1 ▶). ALT levels flared in 11 (37%; 10 genotype 2c) patients who presented with normal or near normal ALT values and in 28 (19%; 21 genotype 2c) patients who had either persistently (more than 18 months) or intermittently high ALT values. Hepatitis reactivated in five patients in the first year of monitoring and in 34 in the subsequent 12–126 months (mean 47 months) of follow up. The rates of ALT flares were 55.6 per 1000 person years for genotype 2c and 15.0 for genotype 1b (p = 0.001), with an overall rate of 34.9. Hepatitis reactivation consisted of 1–4 ALT flares per patient. In 17 patients with genotype 2c, the duration of flare was not evaluable because IFN therapy was started 6–54 months after the increase in ALT. In the 22 untreated patients, the median ALT value during a flare was 460 IU/l (range 201–2200), and flares lasted six months on average. No patient had any increase above 1.5 times the upper normal limits of serum bilirubin or had symptoms of liver disease. The number and severity of flares were similar in the two genotype groups (table 2 ▶).
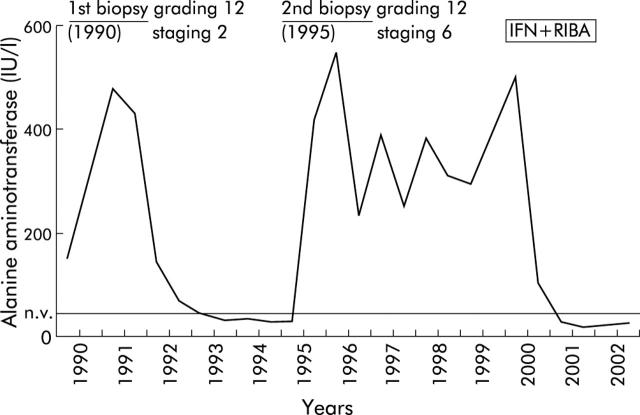
Figure 1.
A 56 year old woman with chronic hepatitis C genotype 2c in whom two alanine aminotransferase flares occurred during follow up, and significant worsening of liver fibrosis was revealed by sequential liver biopsies. The patient achieved a sustained virological response following six months of treatment with interferon IFN α2b (3 MU/three times a week) and ribavirin (1 g/day) (IFN+RIBA).
Table 2.
Incidence rates and severity of hepatitis reactivation
| Hepatitis reactivation | HCV 1b (n = 106) | HCV 2c (n = 100) | All patients (n = 206) |
| Cases | 8a | 31b | 39 |
| Rate×1000 person years | 15.0c | 55.6d | 34.9 |
| No of ALT flares×patient | 1–3e (median 1) | 1–4f (median 1) | 1–4 (median 1) |
| Severity (IU/l) | 201–754g (median 458) | 217–2200h (median 460) | 201–2200 (median 460) |
| Duration (months) | 6–24i (median 6) | 6–90l (median 12) | 6–90 (median 6) |
a versus b, p <0.001.
c versus d, p = 0.001.
e versus f, g versus h, and i versus l, NS.
ALT, alanine aminotransferase; HCV, hepatitis C virus.
After thorough questioning, we were able to rule out an association between hepatitis reactivation and alcohol abuse, or exposure to hepatotoxic drugs or herbal remedies. In all patients, reinfection was excluded on the basis of clinical history and absence of mixed genotypes in serum. Infection with other hepatotropic viruses was ruled out by appropriate serological investigations. By multiple logistic regression analysis, there were no significant associations between the risk of having an ALT flare and patient age or sex, modality or duration of infection, serum ALT values, serum anti-HBc, or histological activity in the diagnostic biopsy (table 3 ▶). Based on the definition of a flare as an ALT value of ⩾400 IU/l, the risk of hepatitis reactivation was 8.32 (95% CI 2.31–29.91) for genotype 2c patients compared with genotype 1b patients. When the definition of a flare was based on a ratio of ⩾8 between the highest and lowest ALT values, the OR of having an ALT flare was 8.00 (95% CI 2.85–22.46) for genotype 2c patients compared with genotype 1b patients. Using either definition, the OR of having an ALT flare was 6.49 (95% CI 2.56–16.44) for genotype 2c patients compared with genotype 1b patients.
Table 3.
Distribution of selected covariates according to alanine aminotransferase (ALT) flare, defined as a maximum value of ⩾400 IU/l or a maximum/minimum ALT ratio value of ⩾8
| Covariates | ALT flare (+):ALT flare (−) | OR* (95% CI) |
| Age (y) | ||
| <45 | 10:47 | 1† |
| 45–49 | 9:31 | 1.34 (0.42–4.28) |
| 50–54 | 8:36 | 1.45 (0.44–4.75) |
| ⩾55 | 12:53 | 1.31 (0.43–3.99) |
| Sex | ||
| Males | 26:94 | 1† |
| Females | 13:73 | 0.59 (0.24–1.44) |
| Modality of infection | ||
| Community acquired | 29:129 | 1† |
| Parenteral | 10:38 | 1.20 (0.40–3.62) |
| Duration of hepatitis (y) | ||
| <10 | 18:79 | 1† |
| 10–19 | 7:35 | 1.00 (0.33–3.06) |
| ⩾20 | 14:53 | 1.22 (0.42–3.54) |
| Basal value of ALT‡ | ||
| <80 | 15:52 | 1† |
| 80–129 | 7:53 | 0.57 (0.17–1.89) |
| ⩾130 | 17:62 | 1.77 (0.61-5.12) |
| Histology grading of baseline biopsy§ | ||
| <8 | 19:84 | 1† |
| 8–10 | 12:51 | 1.79 (0.62–5.11) |
| ⩾11 | 3:18 | 0.84 (0.18–3.87) |
| Missing | 5:14 | – |
| Histology staging of baseline biopsy | ||
| <3 | 25:75 | 1† |
| 3/4 | 5:32 | 0.34 (0.10–1.19) |
| ⩾5 | 4:46 | 0.23 (0.06–0.88) |
| Missing | 5:14 | – |
Odds ratios (ORs) and corresponding 95% confidence intervals (CI).
*Estimates from multiple logistic regression equations, including terms for age, sex, duration of hepatitis C virus, liver histology staging, modality of infection, basal values of ALT, number of observations, and genotype.
†Reference category.
‡Mean of the first three values.
§Not adjusted by liver histology staging.
Changes in liver histology
During the 71 months of observation, 52 patients underwent a second liver biopsy 36–120 months after the diagnostic biopsy. We performed a second liver biopsy in 16 patients who had an ALT flare and in 36 who experienced no flares to assess whether hepatitis had progressed to more severe histological damage that could fulfil indications for IFN therapy. Liver biopsy coincided with a flare in six patients and was delayed for 6–72 months in 10 patients. A total of 135 patients did not undergo a second liver biopsy: 90 patients underwent IFN therapy; 28 had persistently normal or near normal ALT values throughout the observation period, thus not fulfilling the indications for IFN therapy; nine had active cirrhosis; seven did not comply with indications for IFN therapy; and one refused to undergo liver biopsy. Blind assessment of paired biopsies showed no significant changes in grading or staging scores between baseline and follow up liver biopsies in the two genotype groups (6.4 (2.4) and 1.9 (1.7) v 8.6 (2.9) and 3.3 (1.7) for genotype 2c; 6.1 (1.6) and 1.7 (1.1) v 8.0 (1.8) and 2.5 (1.4) for genotype 1b). Analysing hepatitis progression in relation to the presence or absence of an ALT flare, an increase in grading score of 4 points or more was demonstrated in six patients re-biopsied during or after hepatitis reactivation and in nine of those who did not have a flare during the study period (37% v 25%; NS). In contrast, staging score was increased by 2 or more points in 10 patients (63%) in the former group compared with seven (19%) in the latter (p = 0.003). Corresponding mean fibrosis units per year were 0.414 and 0.190, respectively (p = 0.02) (table 4 ▶).
Table 4.
Hepatitis flares and fibrosis progression in chronic hepatitis C patients who underwent repeat liver biopsy
| Histological progression of hepatitis | Patients with an ALT flare (n = 16) | Patients who remained flare free (n = 36) | p Value |
| Grading ⩾4 points | 6 (37%) | 9 (25%) | NS |
| Staging ⩾2 points | 10 (63%) | 7 (19%) | 0.003 |
| Mean fibrosis units per year | 0.414 | 0.190 | 0.02 |
ALT, alanine aminotransferase.
Sixty four patients with genotype 2c and 58 with genotype 1b ultimately received IFN. Twenty patients (31%) in the former group and four (7%) in the latter showed a sustained virological response. In 17 patients, treatment was started after an episode of hepatitis reactivation: nine (53%) had experienced a sustained virological response to therapy.
DISCUSSION
In a previous report18 we described two patients in whom abrupt reactivation of hepatitis C led to liver disease progression after years of subclinical infection with HCV genotype 2c. In this present study, we demonstrated that hepatitis reactivation is part of the natural course of chronic HCV infection and that, at least in Italy, more frequently occurs in carriers of genotype 2c than in those with genotype 1b. As a consequence, genetic heterogeneity of HCV seems to play a role in the evolutionary course of hepatitis C infection.
To date, two major ALT profiles have been described in HCV carriers: persistently normal or near normal ALT values4,24 and persistently or intermittently raised ALT values.1,2 Our study included patients with both ALT profiles but also demonstrated a third pattern in the evolutionary course of HCV infection—that is, an abrupt flare into a more severe clinical condition characterised by ALT peaks of up to 2200 IU/l. The fact that the majority (74%) of patients who developed an ALT flare were older than 45 years suggests that hepatitis C reactivation preferentially occurs late during infection or in aged patients. Previous studies in HCV carriers failed to identify similar episodes of hepatitis reactivation, probably because they applied different criteria for patient selection and follow up.4,25 None of these studies provided a definition of an ALT flare or adequately assessed the frequency of hepatitis reactivation as patients with increases in ALT values were either discharged from follow up or treated with IFN.
The relationship between genotype 2c and risk of hepatitis reactivation calls for refinement of the predictive value of HCV genotypes, at least in our geographical region. Previous studies in patients with chronic hepatitis C failed to demonstrate a correlation between genetic heterogeneity of HCV, ALT levels, and histological severity of hepatitis, probably because heterogeneous criteria for patient recruitment and follow up were applied.8,14,16,26
In this study, hepatitis reactivation appeared to be independent of all other epidemiological and clinical features. Patients who demonstrated hepatitis reactivation lacked serum markers of autoimmunity and showed similar rates of serum markers of occult or previous HBV infection27 as flare free patients. Also, patients who experienced a flare did not report excess consumption of alcohol, drugs, or herbal remedies. Reinfection with HCV as a cause of hepatitis flares was also excluded as all patients lacked any recognisable source of sexual or parenteral exposure and no mixed genotype infections were detected. Due to the close association between genotypes 1a and 3a and intravenous drug injection in Italy,28,29 our criteria for patients selection led to exclusion of illicit drug users who are at high risk of multiple exposures to HCV. Identification of multiple ALT flares in five patients who lacked any recognisable source of infection and/or exposure to non-viral hepatotoxic factors and the high rates of sustained virological responses to IFN in flaring patients reinforced our opinion that hepatitis reactivation is part of the natural course of HCV infection.
Hepatitis C reactivation could be a consequence of loss of host immune control over the virus through multifactorial processes involving time related host changes and genetic diversity of the virus. In fact, HCV genotype 2c has been associated more frequently than genotype 1b with changes over time in the level of the hypervariable region HVR1, which carries epitopes involved in host immune control of HCV.30 Interestingly, hepatitis reactivation is not unique to HCV: carriers of HBeAg (−) variants of HBV, which are common in the Mediterranean basin, were shown to develop abrupt ALT flares and severe fibrosing hepatitis after years of indolent infections.31
The fact that hepatitis reactivation also occurred in carriers with persistently normal or near normal ALT levels and that in some cases it led to significant liver deterioration, further suggests that HCV patients with persistently normal or near normal ALT values are also at risk of progressive fibrosis of the liver.32,33 We acknowledge that our findings should be cautiously interpreted as hepatic fibrosis during an ALT flare may be in part reversible and the number of follow up liver biopsies performed in our patients was limited. We did in fact assess 52 untreated patients as guidelines for good clinical practice did not encourage repetition of liver biopsies in HCV carriers with persistently normal ALT values, patients with histologically proven cirrhosis, and patients who had a sustained virological response to IFN.1
Persistent upgrading of ALT levels observed in several patients after an episode of hepatitis reactivation reinforced our opinion that ALT flares are an unfavourable predictor of hepatitis outcome. Our findings that the evolutionary course of hepatic fibrosis was accelerated in individual patients experiencing an ALT flare challenges the opinion that during hepatitis C, hepatic fibrosis accumulation is linear with time.10,34 When the linear model for predicting the progression of hepatic fibrosis was applied to a small series of infants with chronic hepatitis C, the prediction was unreliable in 97% of cases.35 In an image analysis study aimed at assessing the impact of liver biopsy sampling variability on liver fibrosis evaluation, the increase in fibrous tissue accumulation in hepatitis C was threefold for the early stage F2 samples versus 12-fold for advanced stage F4 samples compared with F0 samples with no fibrosis.36
In conclusion, this study demonstrates that reactivation is part of the natural course of chronic hepatitis C and that it is closely associated with genotype 2c infection. We suggest that genetic heterogeneity of HCV may influence the evolutionary course of hepatitis C infection, further questioning the linearity of fibrosis progression in chronically infected patients.
Acknowledgments
The authors thank Caterina Maria Puricelli for expert secretarial assistance. The study was supported by the MURST EX-40% 2000, FIRST 2001, and CNR 99.02432.CT04, and the Italian Foundation for Cancer Research and the Italian Association for Cancer Research (FIRC/AIRC)
Abbreviations
IFN, interferon
HBsAg, hepatitis B surface antigen
ALT, alanine aminotransferase
AST, aspartate aminotransferase
HAV, hepatitis A virus
HCV, hepatitis C virus
HBV, hepatitis B virus
CMV, cytomegalovirus
EBV, Epstein-Barr virus
HVS1 and HVS2, herpes viruses
anti-HBc, antibody to hepatitis B core antigen
RT-PCR, nested reverse transcription-polymerase chain reaction
OR, odds ratio
Conflict of interest: None declared.
REFERENCES
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41–52. [DOI] [PubMed] [Google Scholar]
- 2.Poynard T, Yuen MF, Ratziu V, et al. Viral hepatitis C. Lancet 2003;362:2095–100. [DOI] [PubMed] [Google Scholar]
- 3.Kenny-Walsh E for the Irish Hepatology Research Group. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N Engl J Med 1999;340:1228–33. [DOI] [PubMed] [Google Scholar]
- 4.Persico M, Persico E, Suozzo R, et al. Natural history of hepatitis C virus carriers with persistently normal aminotransferase levels. Gastroenterology. 2000;118;760–4. [DOI] [PubMed]
- 5.Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med 2000;132:105–11. [DOI] [PubMed] [Google Scholar]
- 6.Martinot-Peignoux M, Boyer N, Cazals-Hatem D, et al. Prospective study on anti-hepatitis C virus-positive patients with persistently normal serum alanine transaminase with or without detectable serum hepatitis C virus RNA. Hepatology 2001;34:1000–5. [DOI] [PubMed] [Google Scholar]
- 7.Tong MJ, El-Farra NS, Reikes AR, et al. Clinical outcomes after transfusion associated hepatitis C. N Engl J Med 1995;332:1463–6. [DOI] [PubMed] [Google Scholar]
- 8.Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology 1998;28:1687–95. [DOI] [PubMed] [Google Scholar]
- 9.Datz C, Cramp M, Haas T, et al. The natural course of hepatitis C virus infection 18 years after an epidemic outbreak of non-A, non-B hepatitis in a plasmapheresis centre. Gut 1999;44:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poynard T, Bedossa P, Opolon P, et al. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 1997;349:825–32. [DOI] [PubMed] [Google Scholar]
- 11.Puoti M, Bonacini M, Spinetti A, et al. Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J Infect Dis 2001;183:134–7. [DOI] [PubMed] [Google Scholar]
- 12.Goedert JJ, Eyster ME, Lederman MM, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood 2002;100:1584–9. [PubMed] [Google Scholar]
- 13.Nousbaum JB, Pol S, Nalpas B, et al. Hepatitis C virus type 1b (II) infection in France and Italy. Ann Intern Med 1995;122:161–8. [DOI] [PubMed] [Google Scholar]
- 14.Romeo R, Rumi MG, Del Ninno E, et al. Hepatitis C virus genotype 1b and risk of hepatocellular carcinoma. Hepatology 1997;26:1077. [DOI] [PubMed] [Google Scholar]
- 15.Bruno S, Silini E, Crosignani A, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a prospective study. Hepatology 1997;25:754–8. [DOI] [PubMed] [Google Scholar]
- 16.Serfaty L, Aumaitre H, Chazouilléres O, et al. Determinants of outcome of compensated hepatitis C virus-related cirrhosis. Hepatology 1998;27:1435–40. [DOI] [PubMed] [Google Scholar]
- 17.Strader DB, Wright T, Thomas DL, et al. Diagnosis management and treatment of hepatitis C. Hepatology 2004;39:1147–71. [DOI] [PubMed] [Google Scholar]
- 18.Rumi MG, De Filippi F, Donato MF, et al. Progressive hepatic fibrosis in healthy carriers of hepatitis C virus with a transaminase breakthrough. J Viral Hepat 2001;9:71–4. [DOI] [PubMed] [Google Scholar]
- 19.Rumi MG, Del Ninno E, Parravicini ML, et al. A prospective randomized trial comparing lymphoblastoid to recombinant interferon alfa 2a as therapy for chronic hepatitis C. Hepatology 1996;24:1366–70. [DOI] [PubMed] [Google Scholar]
- 20.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–9. [DOI] [PubMed] [Google Scholar]
- 21.Silini E, Bono F, Cividini A, et al. Differential distribution of hepatitis C virus genotypes in patients with and without liver function abnormalities. Hepatology 1995;21:285–90. [PubMed] [Google Scholar]
- 22.Cammarota G, Maggi F, Vatteroni ML, et al. Partial nucleotide sequencing of six subtype 2c hepatitis C viruses detected in Italy. J Clin Microbiol 1995;33:2781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breslow NE, Day NE. Statistical methods in cancer research, vol. 1. The analysis of case-control studies. IARC Scientific Publications No 32. Lyon: IARC, 1980. [PubMed]
- 24.Puoti C, Magrini A, Stati T, et al. Clinical, histological and virological features of hepatitis c virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology 1997;26:1393–8. [DOI] [PubMed] [Google Scholar]
- 25.Puoti C, Castellacci R, Montagnese F, et al. Histological and virological features and follow-up of hepatitis C virus carriers with normal aminotransferase levels: the Italian prospective study of the asymptomatic C carriers (ISACC). J Hepatol 2002;37:117–23. [DOI] [PubMed] [Google Scholar]
- 26.Benvegnù L, Pontisso P, Cavalletto D, et al. Lack of correlation between hepatitis C virus genotypes and clinical course of hepatitis C virus-related cirrhosis. Hepatology 1997;25:211–15. [DOI] [PubMed] [Google Scholar]
- 27.Cacciola I, Pollicino T, Squadrito G, et al. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med 1999;341:22–6. [DOI] [PubMed] [Google Scholar]
- 28.Ravaggi A, Zonaro A, Marin MG, et al. Distribution of viral genotypes in Italy determined by hepatitis C virus typing by DNA immunoassay. J Clin Microbiol 1994;32:2280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pontisso P, Ruvoletto MG, Nicoletti M, et al. Distribution of three major hepatitis C virus genotype in Italy. A multicentre study of 495 patients with chronic hepatitis C. J Viral Hepat 1995;2:33–8. [DOI] [PubMed] [Google Scholar]
- 30.Brambilla S, Bellati G, Asti M, et al. Dynamics of hypervariable region 1 variation in hepatitis C virus infection and correlation with clinical and virological features of liver disease. Hepatology 1998;27:1678–86. [DOI] [PubMed] [Google Scholar]
- 31.Hadzyiannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology 2001;34:617–24. [DOI] [PubMed] [Google Scholar]
- 32.Mathurin P, Moussalli J, Cadranel JF, et al. Slow progression rate of fibrosis in hepatitis C virus patients with persistently normal alanine transaminase activity. Hepatology 1998;27:868–72. [DOI] [PubMed] [Google Scholar]
- 33.Alberti A, Noventa F, Benvegnù L, et al. Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection. Ann Intern Med 2002;137:961–4. [DOI] [PubMed] [Google Scholar]
- 34.Poynard T, Ratziu V, Charlotte F, et al. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol 2001;34:730–9. [DOI] [PubMed] [Google Scholar]
- 35.Guido M, Bortolotti F, Leandro G, et al. Fibrosis in chronic hepatitis C acquired in infancy: is it only a matter of time? Am J Gastroenterol 2003;98:660–3. [DOI] [PubMed] [Google Scholar]
- 36.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449–57. [DOI] [PubMed] [Google Scholar]