Figure 3.
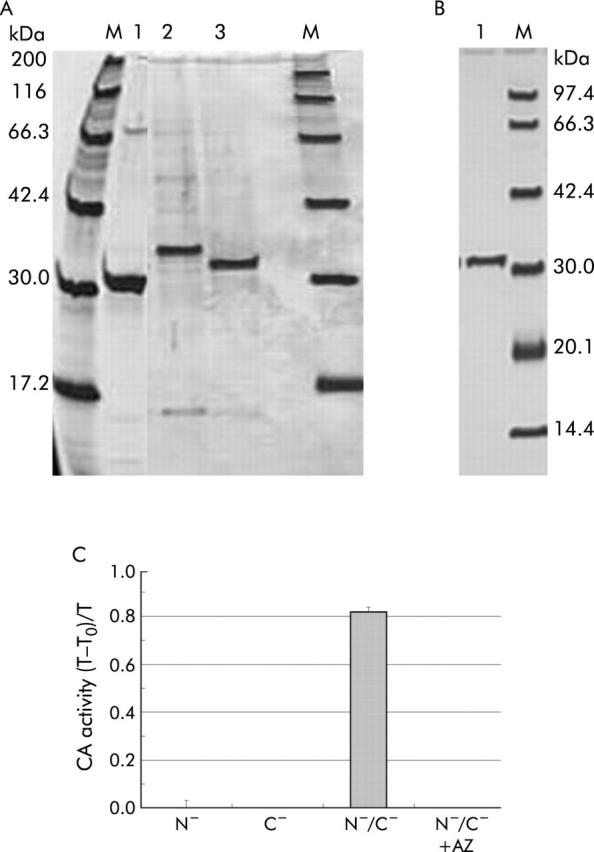
Molecular properties of three truncated carbonic anhydrase (CA) IV proteins. (A) A silver stained sodium dodecyl sulphate-polyacrylamide gel electrophoresis profile with three types of truncated CA IV after denaturing and refolding. Along with several faint bands, a strongly expressed protein was seen in each lane, corresponding to N−/C−-CA IV (30.3 kDa; lane 1), N−-CA IV (33.2 kDa; lane 2), and C−-CA IV (32.2 kDa; lane 3). (B) A silver stained sodium dodecyl sulphate-polyacrylamide gel electrophoresis profile with the N−/C−-CA IV protein, which was further purified with the use of an N-hydroxysuccinimide activated affinity column. A single band was observed and was then considered as an antigen for ELISA. (C) Catalytic activity of three truncated CA proteins: T-T0/T, time to reach equilibration in the CO2 hydration reaction in the absence of CA (T0) and in the presence of a sample (T). Only the N−/C−-CA IV protein showed CA activity (82% of T-T0/T). CA activity was completely inhibited by acetazolamide (AZ).
