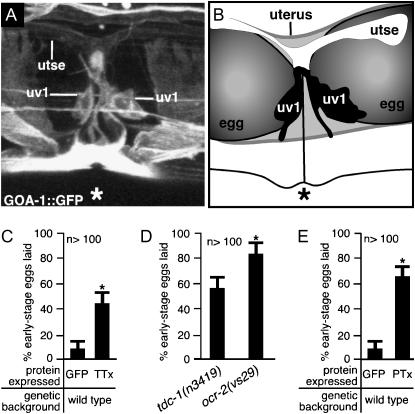
Figure 7.—
uv1 cells release neurotransmitters and require the G-protein GOA-1 to inhibit egg laying until the uv1 cells are mechanically deformed. (A and B) uv1 cells are deformed by eggs in the uterus. The uv1 cells, utse, and the vulva (*) are indicated. (A) A full-length GOA-1∷GFP fluorescent reporter is expressed strongly in uv1, weakly in utse, and in nearby cells, allowing visualization of anatomy in living animals. This projection of a three-dimensional confocal image is shown in various rotations in supplemental Figure S2 at http://www.genetics.org/supplemental/. (B) Tracing from the fluorescence image shown in A and a corresponding bright-field image showing the uv1 cells, utse, and eggs in the uterus. (C) Blocking neurotransmission in ocr-2-expressing cells causes premature egg laying. The ocr-2 promoter and the 3′ regulatory region were used either to express the light chain of tetanus toxin (TTx) to block neurotransmission or to express control GFP. Error bars indicate 95% confidence intervals, and asterisks denote significant differences (P < 0.05) in C–E. (D) ocr-2(vs29) animals have a more severe premature egg-laying defect than do animals lacking TDC-1, the biosynthetic enzyme for tyramine. (E) Inactivating the G-protein GOA-1 in ocr-2-expressing cells causes premature egg laying. The ocr-2 promoter and the 3′ regulatory region were used either to express the S1 subunit of pertussis toxin (PTx) to inactivate GOA-1 or to express control GFP (data replotted from C).