Abstract
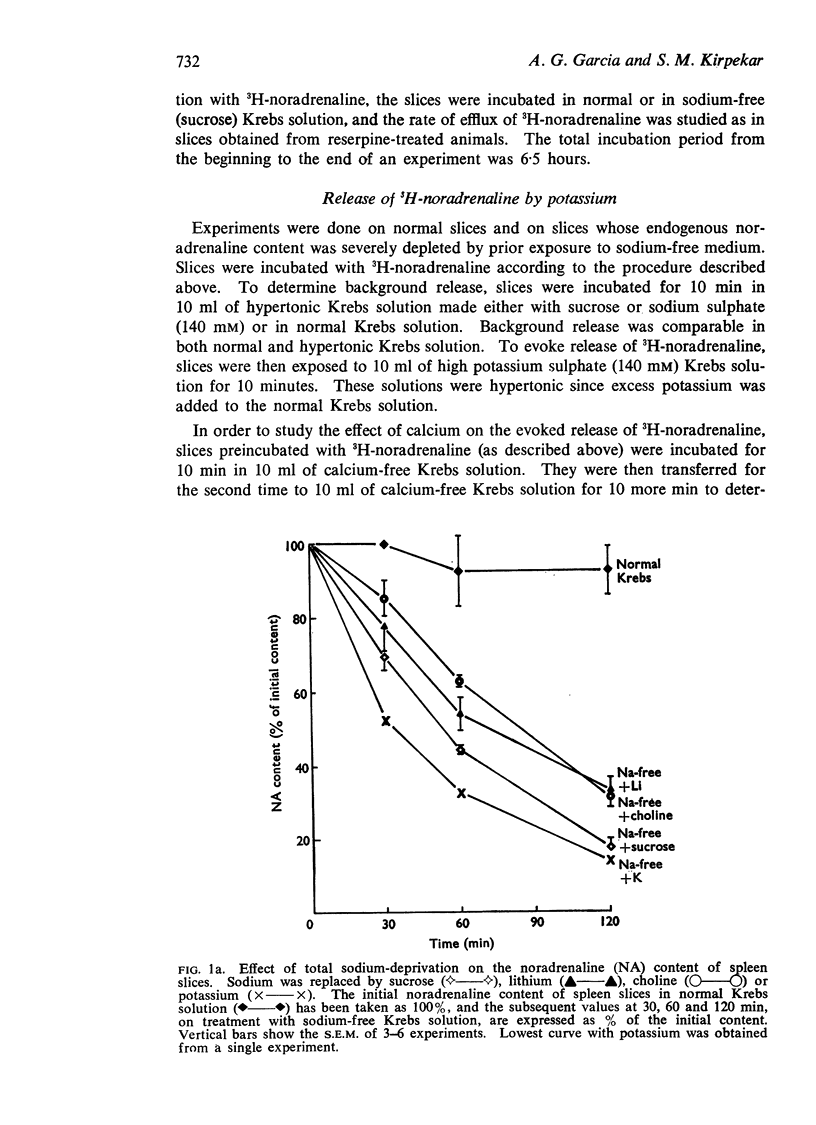
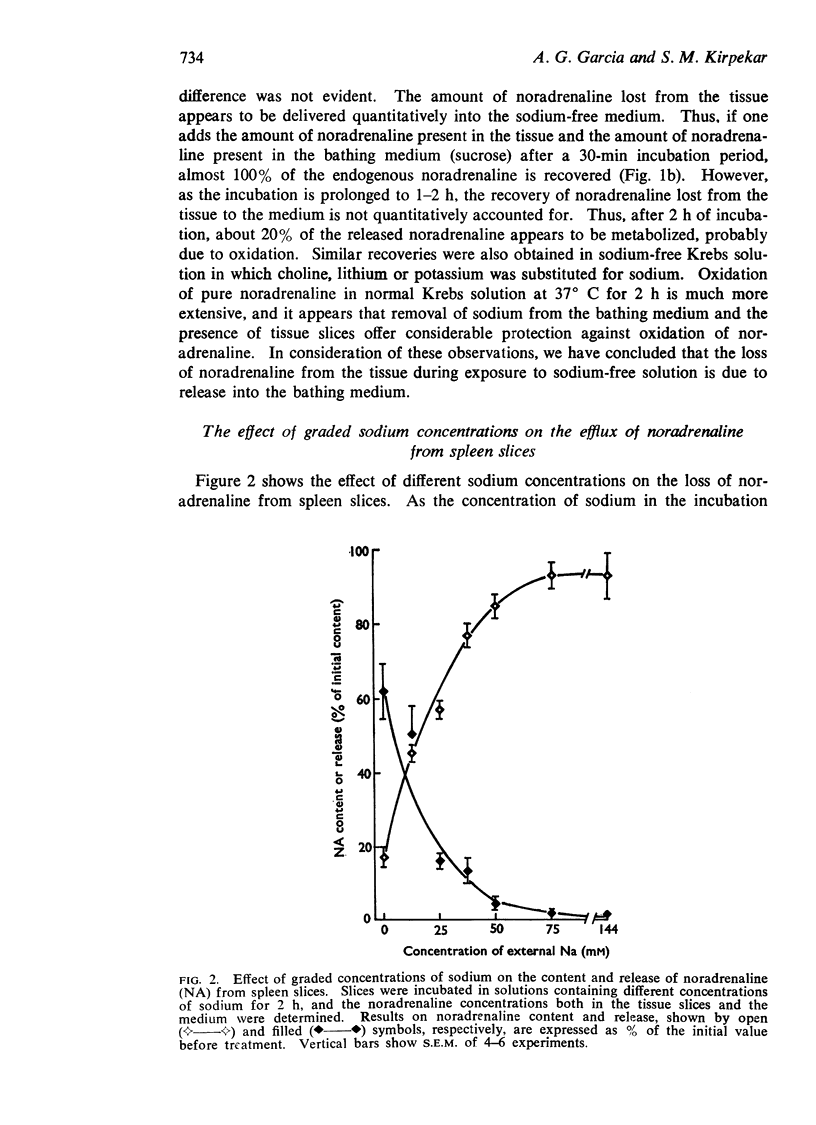
1. The endogenous noradrenaline content of cat spleen slices was markedly reduced when the slices were incubated at 37° C in a medium in which sodium was replaced by sucrose, lithium, choline or potassium. Depletion of tissue noradrenaline was accounted for by its release into the incubating medium. At an external sodium concentration of 20 mM, about 50% depletion was obtained in 2 hours.
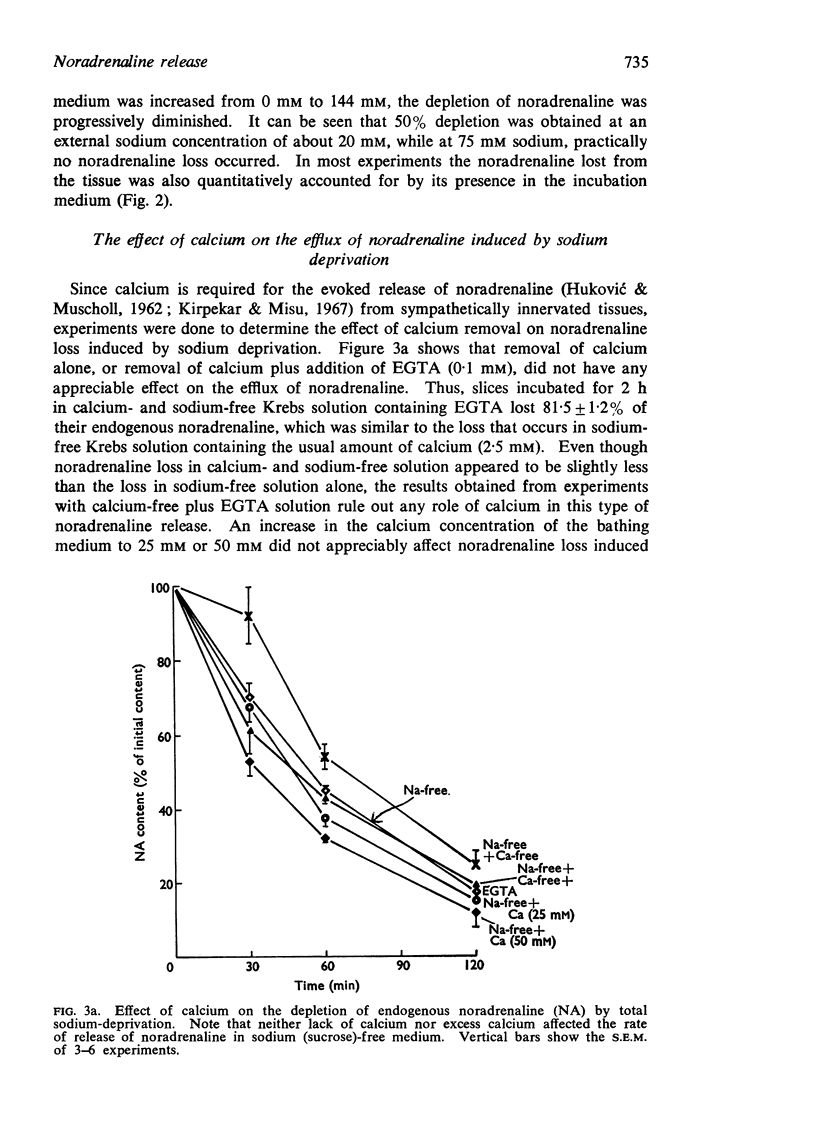
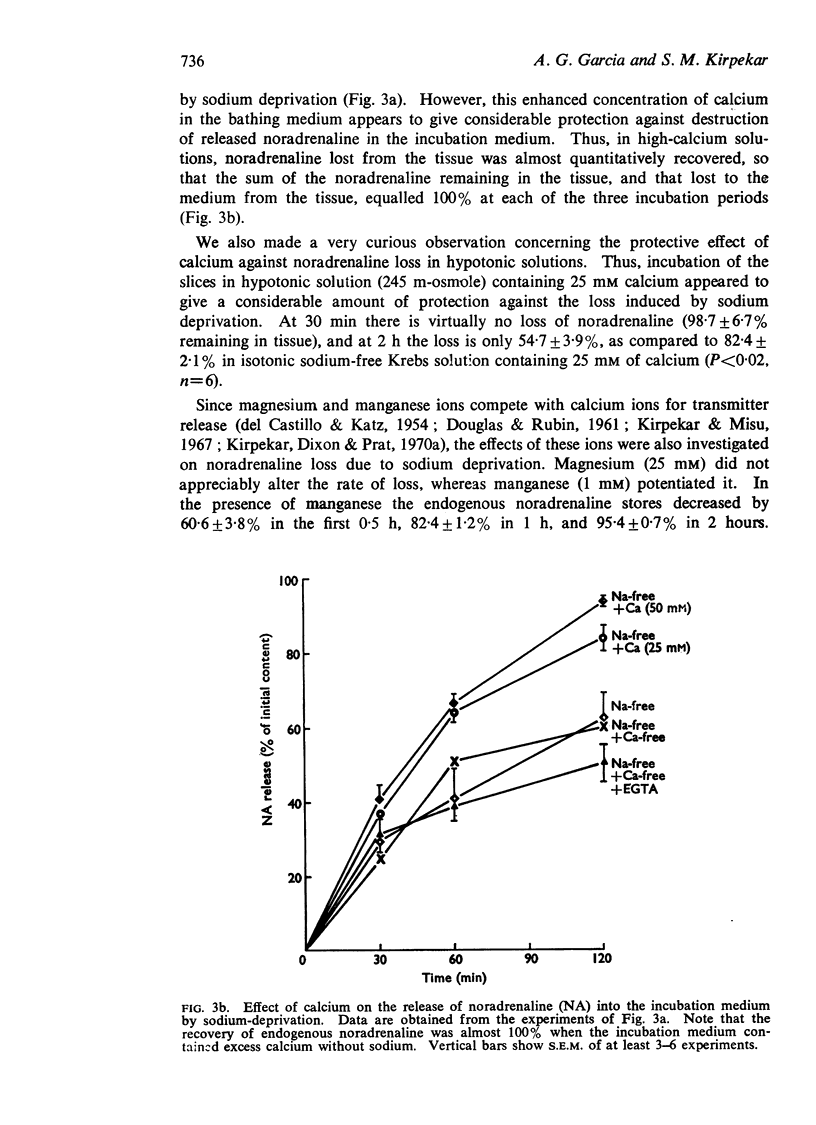
2. The enhanced release induced by sodium deprivation occurred in the absence of calcium, with or without ethyleneglycol-bis (β-aminoethyl ether) N,N′ tetraacetic acid. Manganese potentiated release, while magnesium was without effect.
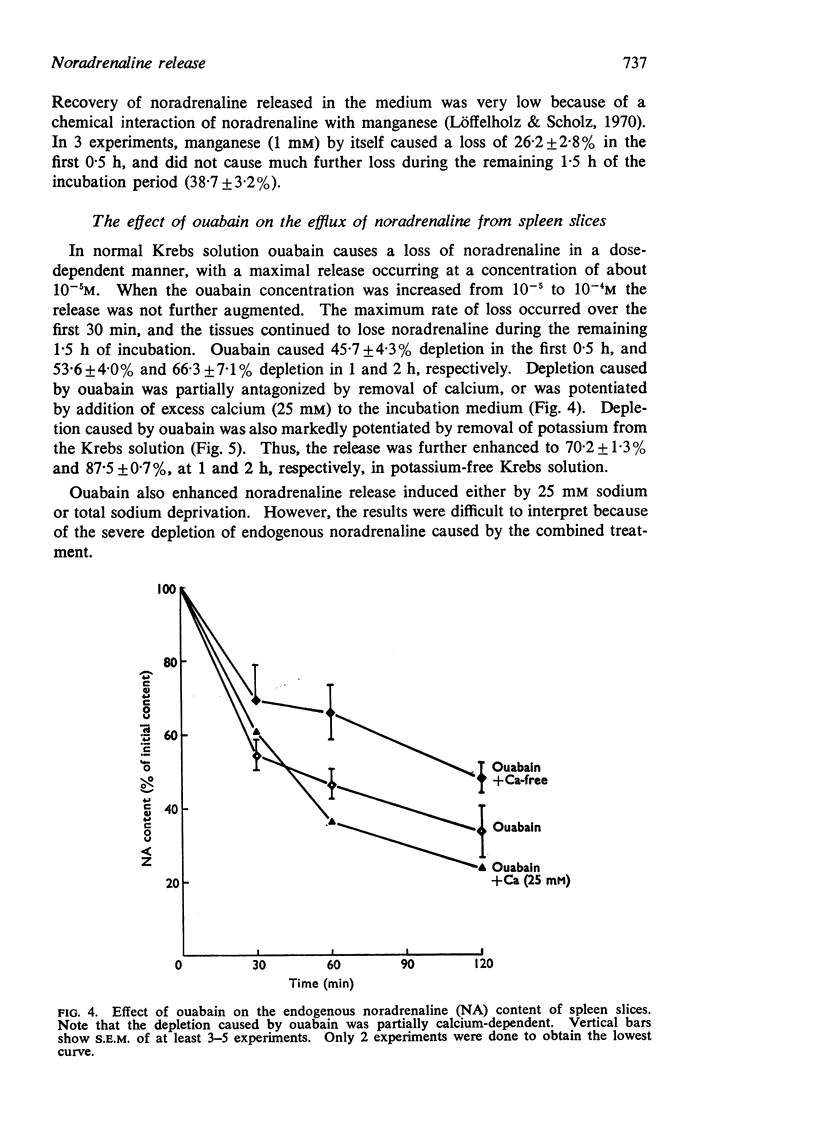
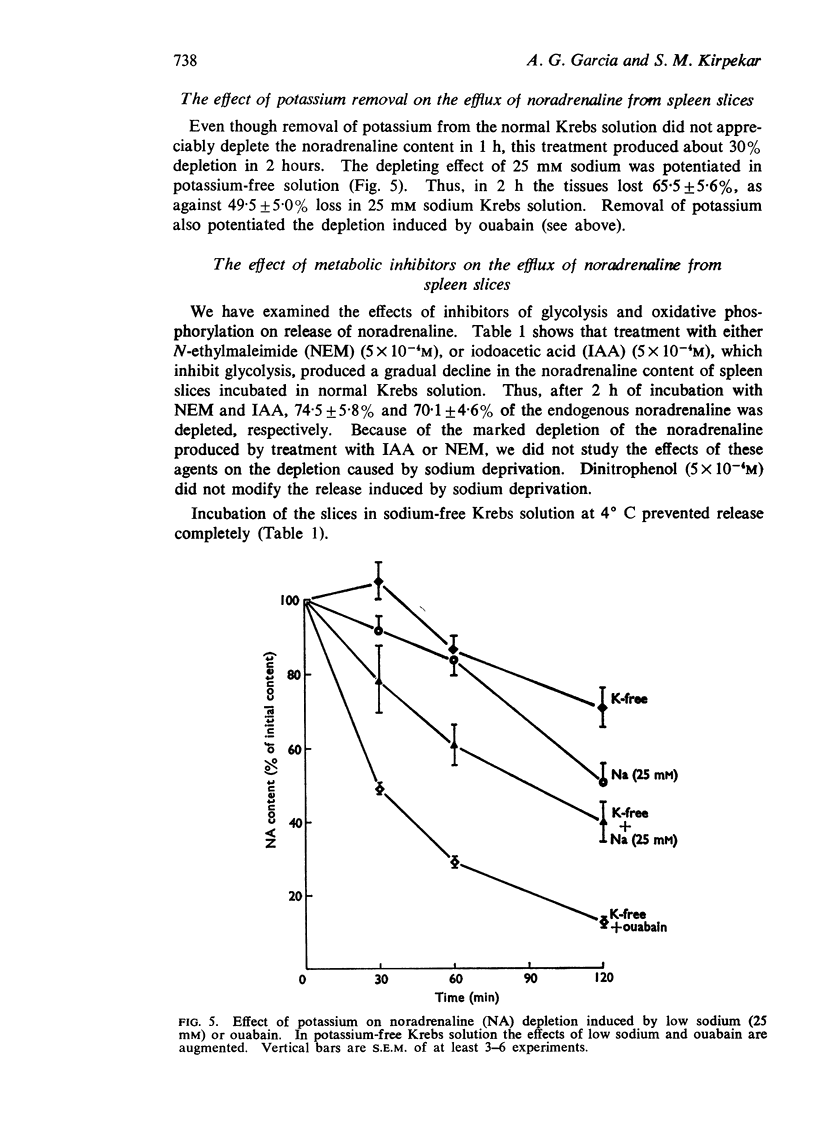
3. Ouabain caused a dose-dependent release of noradrenaline which was partially calcium-dependent. Removal of potassium from the incubation medium caused some release, which was potentiated in 25 mM sodium Krebs solution or by ouabain.
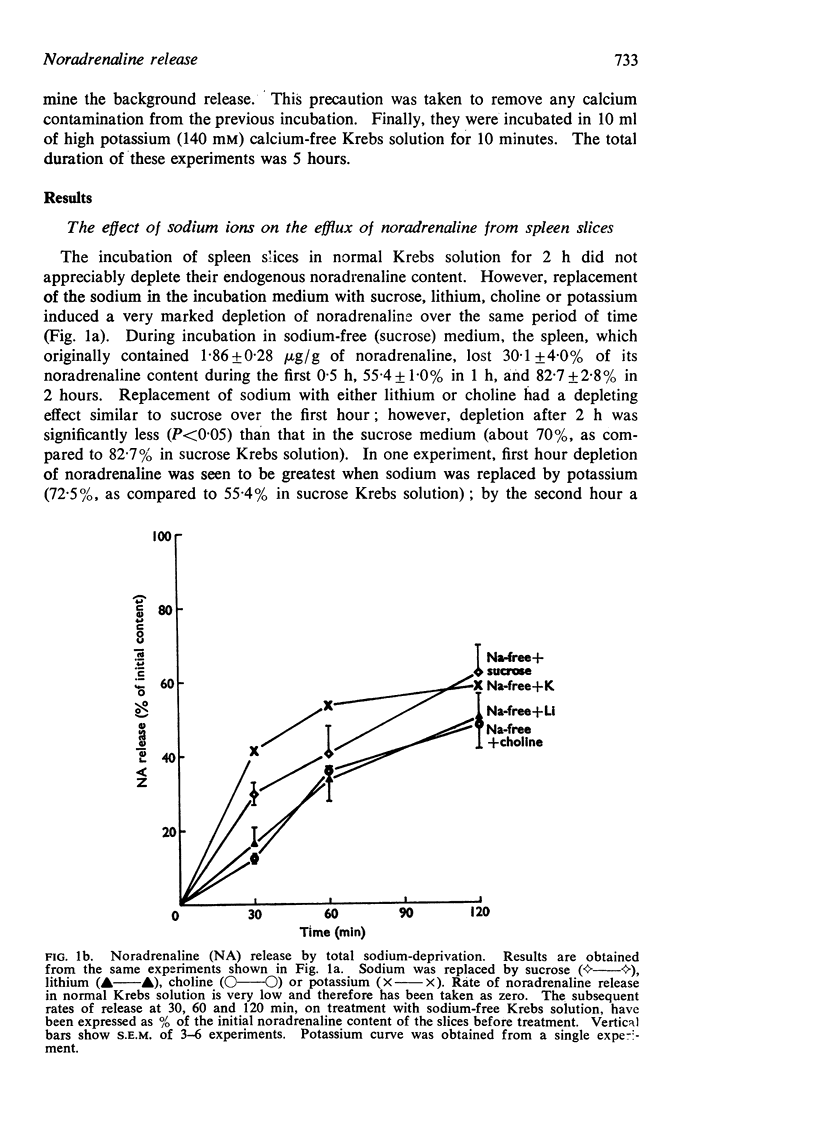
4. At 4° C, the release did not occur in sodium-free medium.
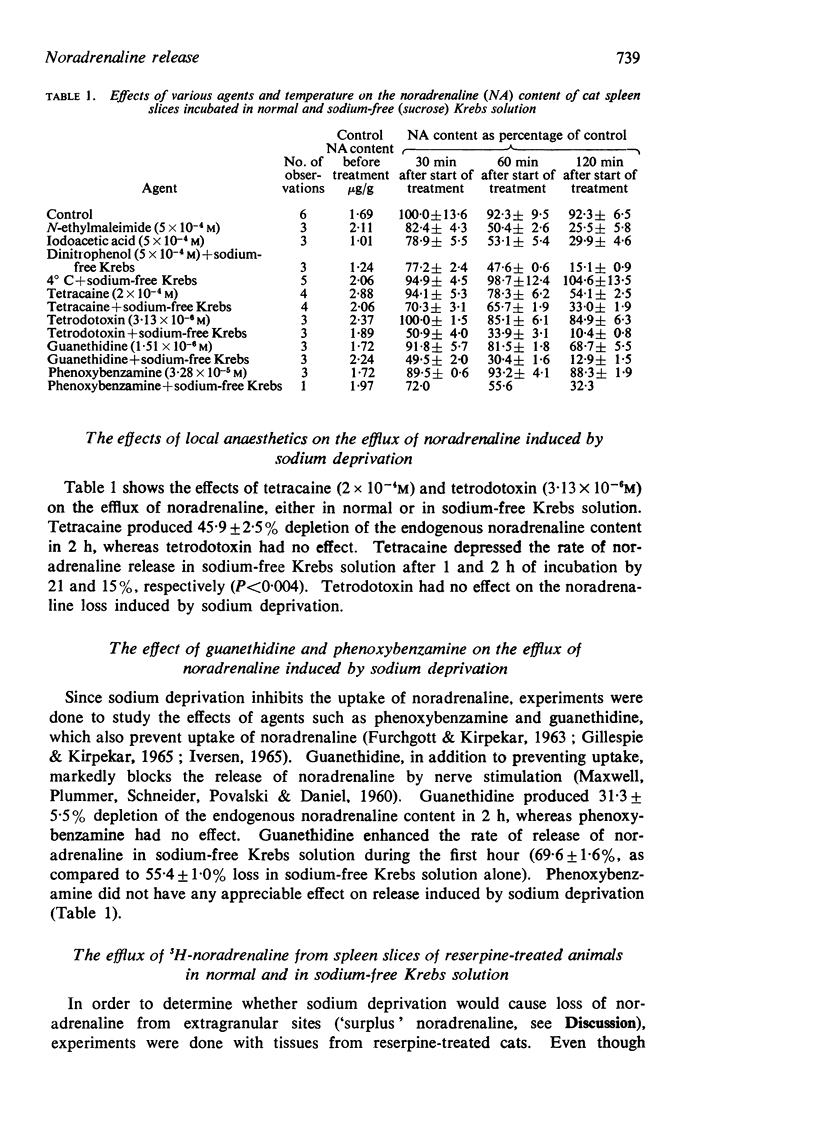
5. Dinitrophenol did not affect the loss of noradrenaline caused by sodium withdrawal. Iodoacetic acid and N-ethylmaleimide caused a time-dependent depletion of noradrenaline. Tetracaine caused release and partly opposed the release caused by sodium deprivation. Tetrodotoxin had no effect. Guanethidine, but not phenoxybenzamine, released noradrenaline and potentiated the release induced by sodium withdrawal.
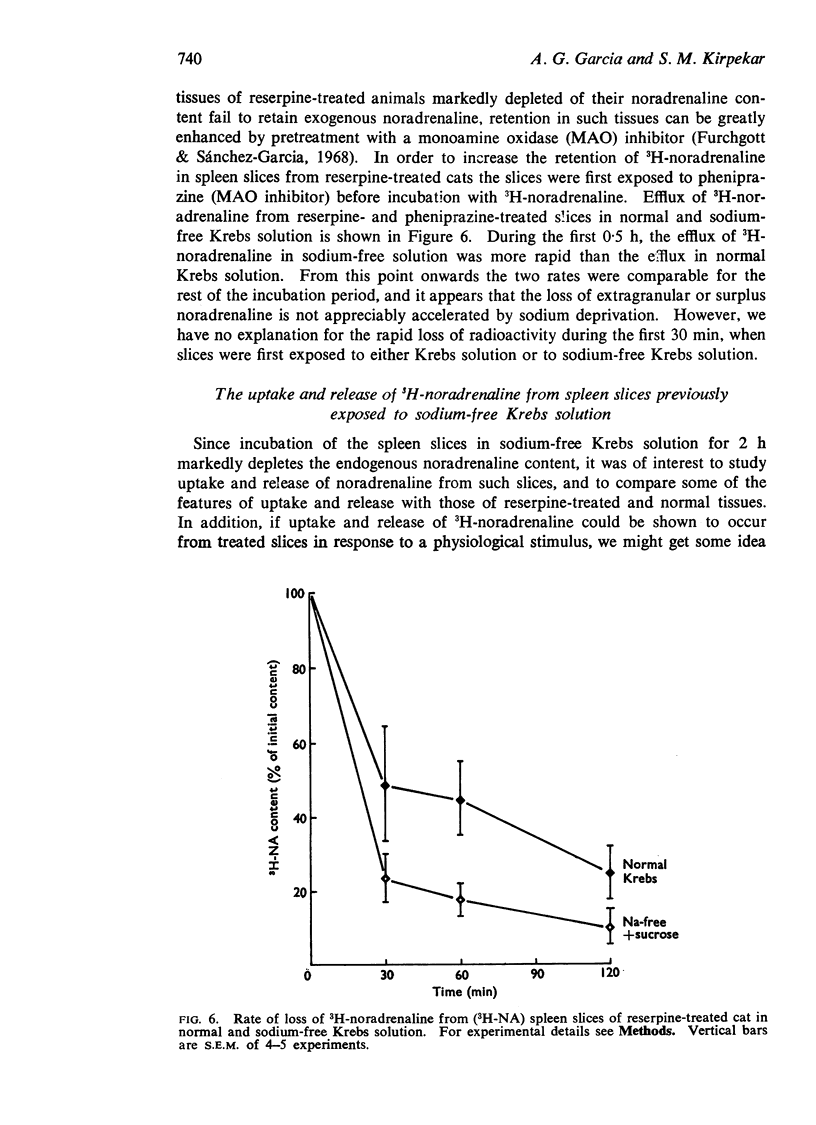
6. The rate of release of 3H-noradrenaline from reserpine-treated spleen slices was not altered by sodium withdrawal.
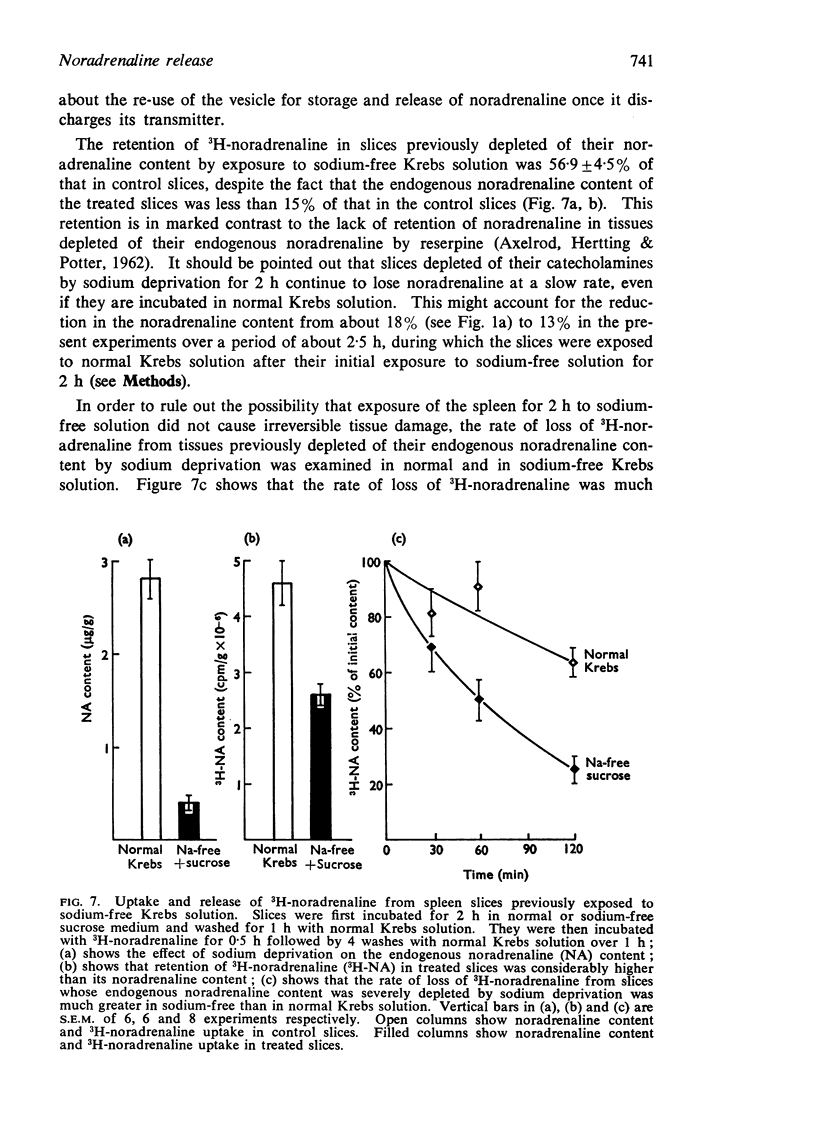
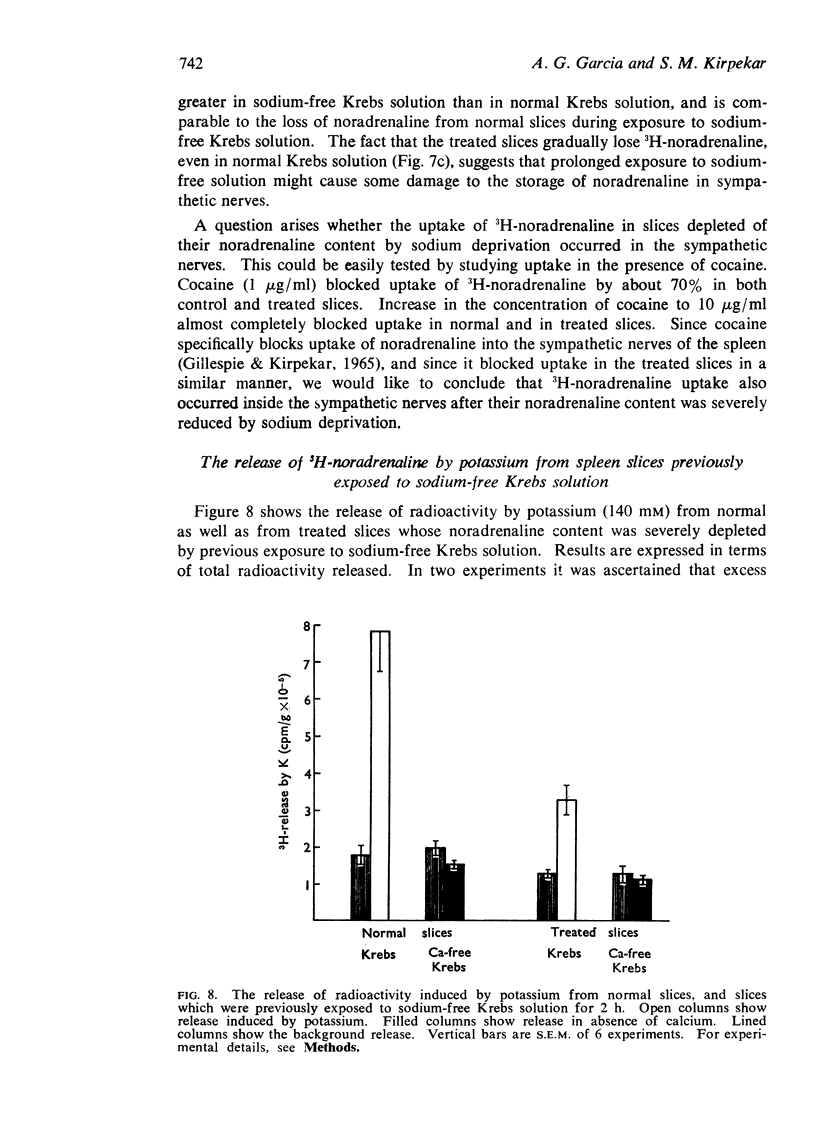
7. Uptake-retention of 3H-noradrenaline in slices depleted of their endogenous noradrenaline content by sodium deprivation was about 60% of the control slices. This was effectively blocked by cocaine. Release of 3H-noradrenaline evoked by high potassium from both control and treated slices was calcium-dependent.
8. It is suggested that sodium-potassium-activated ATPase maintains the integrity of the axonal membrane, and any procedure which depresses the activity of the enzyme or the sodium-potassium pump would cause transmitter release by causing temporary disturbance in the membrane. Evidence is presented to suggest that vesicles depleted of their endogenous noradrenaline content by sodium deprivation are re-used for the storage and release of transmitter.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- AXELROD J., HERTTING G., POTTER L. Effect of drugs on the uptake and release of 3H-norepinephrine in the rat heart. Nature. 1962 Apr 21;194:297–297. doi: 10.1038/194297a0. [DOI] [PubMed] [Google Scholar]
- BONTING S. L., CARAVAGGIO L. L., HAWKINS N. M. Studies on sodium-potassium-activated adenosinetriphosphatase. IV. Correlation with cation transport sensitive to cardiac glycosides. Arch Biochem Biophys. 1962 Sep;98:413–419. doi: 10.1016/0003-9861(62)90206-0. [DOI] [PubMed] [Google Scholar]
- Banerjee S. P., Wong S. M., Khanna V. K., Sen A. K. Inhibition of sodium- and potassium-dependent adenosine triphosphatase by N-ethylmaleimide. I. Effects on sodium-sensitive phosphorylation and potassium-sensitive dephosphorylation. Mol Pharmacol. 1972 Jan;8(1):8–17. [PubMed] [Google Scholar]
- Birks R. I., Burstyn P. G., Firth D. R. The form of sodium-calcium competition at the frog myoneural junction. J Gen Physiol. 1968 Dec;52(6):887–907. doi: 10.1085/jgp.52.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Blaszkowski T. P., Bogdanski D. F. Possible role of sodium and calcium ions in retention and physiological release of norepinephrine by adrenergic nerve endings. Biochem Pharmacol. 1971 Dec;20(12):3281–3294. doi: 10.1016/0006-2952(71)90433-3. [DOI] [PubMed] [Google Scholar]
- Bogdanski D. F., Brodie B. B. The effects of inorganic ions on the storage and uptake of H3-norepinephrine by rat heart slices. J Pharmacol Exp Ther. 1969 Feb;165(2):181–189. [PubMed] [Google Scholar]
- Boullin D. J. The action of extracellular cations on the release of the sympathetic transmitter from peripheral nerves. J Physiol. 1967 Mar;189(1):85–99. doi: 10.1113/jphysiol.1967.sp008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J. H., Gibbons W. R. The release of noradrenaline from sympathetic fibres in relation to calcium concentration. J Physiol. 1965 Nov;181(1):214–223. doi: 10.1113/jphysiol.1965.sp007756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F., KIRPEKAR S. M., RIEKER M., SCHWAB A. ACTIONS AND INTERACTIONS OF NOREPINEPHRINE, TYRAMINE AND COCAINE ON AORTIC STRIPS OF RABBIT AND LEFT ATRIA OF GUINEA PIG AND CAT. J Pharmacol Exp Ther. 1963 Oct;142:39–58. [PubMed] [Google Scholar]
- Furchgott R. F., Sanchez Garcia P. Effects of inhibition of monoamine oxidase on the actions and interactions of norepinephrine, tyramine and other drugs on guinea-pig left atrium. J Pharmacol Exp Ther. 1968 Sep;163(1):98–122. [PubMed] [Google Scholar]
- GILLESPIE J. S., KIRPEKAR S. M. THE INACTIVATION OF INFUSED NORADRENALINE BY THE CAT SPLEEN. J Physiol. 1965 Jan;176:205–227. doi: 10.1113/jphysiol.1965.sp007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis C. N., Paton D. M. Cation dependence of sympathetic transmitter retention by slices of rat ventricle. Br J Pharmacol Chemother. 1967 Mar;29(3):309–318. doi: 10.1111/j.1476-5381.1967.tb01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUKOVIC S., MUSCHOLL E. [Noradrenalin loss from the isolated rabbit heart following sympathetic nerve irritation and its pharmacological alteration]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1962;244:81–96. [PubMed] [Google Scholar]
- Iversen L. L., Kravitz E. A. Sodium dependence of transmitter uptake at adrenergic nerve terminals. Mol Pharmacol. 1966 Jul;2(4):360–362. [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen P. M., Bogdanski D. F. Sodium and calcium ions in uptake and release of norepinephrine by nerve endings. Am J Physiol. 1970 Sep;219(3):677–682. doi: 10.1152/ajplegacy.1970.219.3.677. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Dixon W., Prat J. C. Inhibitory effect of manganese on norepinephrine release from the splenic nerves of cats. J Pharmacol Exp Ther. 1970 Jul;174(1):72–76. [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Yamamoto H. Effects of metabolic inhibitors on norepinephrine release from the perfused spleen of the cat. J Pharmacol Exp Ther. 1970 Apr;172(2):342–350. [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R., Dixon W., Prat J. C. Effect of cocaine, phenoxybenzamine and calcium on the inhibition of norepinephrine output from the cat spleen by guanthidine. J Pharmacol Exp Ther. 1969 Feb;165(2):166–175. [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Factors influencing noradrenaline uptake by the perfused spleen of the cat. J Physiol. 1968 Feb;194(3):609–626. doi: 10.1113/jphysiol.1968.sp008428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Release of noradrenaline from the cat spleen by potassium. J Physiol. 1968 Feb;194(3):595–608. doi: 10.1113/jphysiol.1968.sp008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffelholz K., Scholz H. Inhibition of Mn plus plus-catalyzed autoxidation of adrenaline by ascorbic acid. Experientia. 1970 Jun 15;26(6):637–638. doi: 10.1007/BF01898734. [DOI] [PubMed] [Google Scholar]
- MAXWELL R. A., PLUMMER A. J., SCHNEIDER F., POVALSKI H., DANIEL A. I. Pharmacology of [2-(octahydro-1-azocinyl)-ethyl]-guanidine sulfate (Su-5864). J Pharmacol Exp Ther. 1960 Jan;128:22–29. [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S., Zar M. A. The mechanism of acetylcholine release from parasympathetic nerves. J Physiol. 1971 Jul;215(3):819–848. doi: 10.1113/jphysiol.1971.sp009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendi R. Sodium, potassium-requiring adenosinetriphosphatase activity. II. Mechanism of inhibition by sulphydryl reagents. Biochim Biophys Acta. 1965 Jun 22;99(3):564–566. doi: 10.1016/s0926-6593(65)80215-6. [DOI] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- SKOU J. C. Studies on the Na ion and K ion activated ATP hydrolysing enzyme system. The role of SH groups. Biochem Biophys Res Commun. 1963 Jan 18;10:79–84. doi: 10.1016/0006-291x(63)90272-9. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Huerlimann A., Haefely W. Interaction of phenoxybenzamine with guanethidine and bretylium at the sympathetic nerve endings of the isolated perfused spleen of the cat. J Pharmacol Exp Ther. 1966 Feb;151(2):189–195. [PubMed] [Google Scholar]
- Wakade A. R., Furchgott R. F. Metabolic requirements for the uptake and storage of norepinephrine by the isolated left atrium of the guinea pig. J Pharmacol Exp Ther. 1968 Sep;163(1):123–135. [PubMed] [Google Scholar]


