Figure 2.
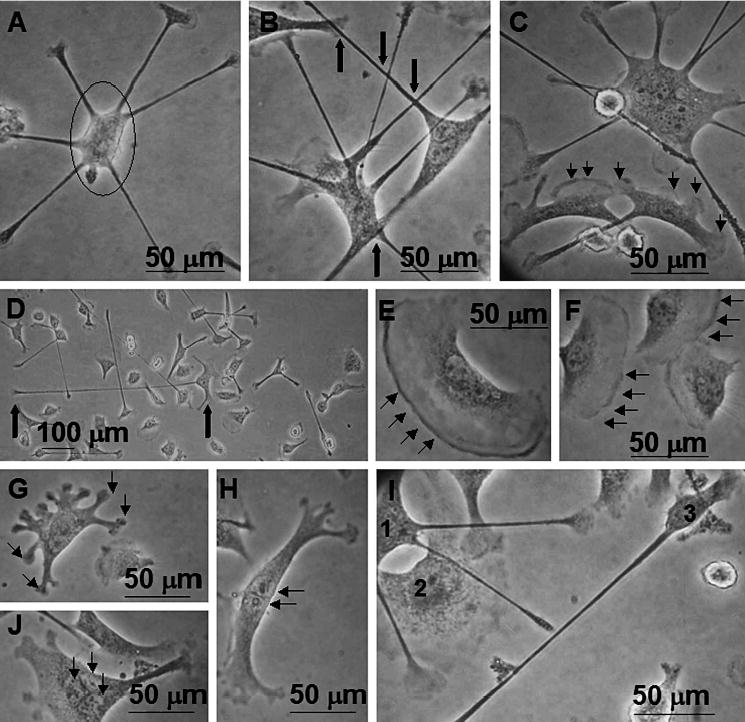
Phase contrast photomicrographs of IC-21 macrophage morphologies on Teflon® AF surfaces. (A) Cells commonly exhibit loosely adherent cell bodies (circle) with numerous filopodia, sometimes in a circular arrangement. (B) Distinct from cultures on all other surface chemistries, filopodial extensions are often observed to cross one another (bold arrows), or to terminate in proximity to attachment sites of other cells. (C) Cell populations on Teflon® AF surfaces show a mixture of actin-based cytoskeletal features (filopodia, lamellipodia, membrane ruffling), often with numerous features on a single cell, as illustrated by all 3 cells in this field. Note differences in cell size, shape and features. (D) Filopodia are frequently lengthy, extending hundreds of μm from the cell body (bold arrows indicate cell body (right) and filopodium terminus (left)). (E, F) Cells showing characteristic membrane ruffling, indicative of the ‘lagging edge’ of a motile cell. (G, H) Less typical morphologies observed on Teflon® AF include cells with numerous bulbous attachment sites (G) and tree-trunk-like morphologies (H). (I) This field illustrates the diversity of the cell population observed: cell ‘1’ has multiple lengthy filopodia that cross over cell ‘2’, and that are diffuse rather than punctate at their termini, possibly indicative of active surface probing. Cell ‘2’ has a diffuse cytoplasm, with large areas if membrane ruffling. Cell ‘3’ has a small well-defined cell body with one lengthy filopodium. (J) Occasionally, cells with multiple nuclei (3–5) have been observed on Teflon® AF without the addition of exogenous cytokines that promote fusion events. Results are representative of numerous (>3) experiments.
