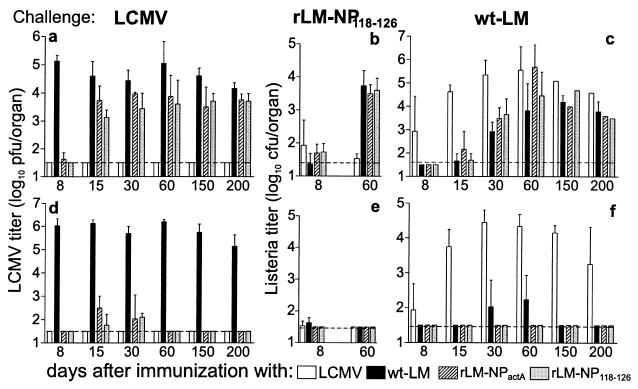
Figure 2.
In vivo protection against a challenge infection with LCMV, rLM, and wt-LM after vaccination with recombinant Listeria. BALB/c mice were immunized with 200 pfu of LCMV or 2 × 103 cfu of Listeria (wt-LM; rLM-NPactA; rLM-NP118–126). Memory mice were challenged after the time points indicated with 200 pfu of LCMV (a and d), 104 cfu of rLM-NP118–126 (b and e), or104 cfu of wt-LM (c and f) intravenously. The viral and bacterial titers in the spleen and the liver were determined 36 hours (a–c) and 5 days (d–f) later. The horizontal dashed line indicates the detection limit. The mean ± SD of three mice per group is given. Naive mice challenged with 104cfu of Listeria had bacterial titers in the spleen of 6.5 ± 0.1 cfu after 36 hours, 4.7 ± 0.9 cfu at day 5. Only bacterial titers in the spleen are shown in the graph; Listeria titers in the liver were comparable.