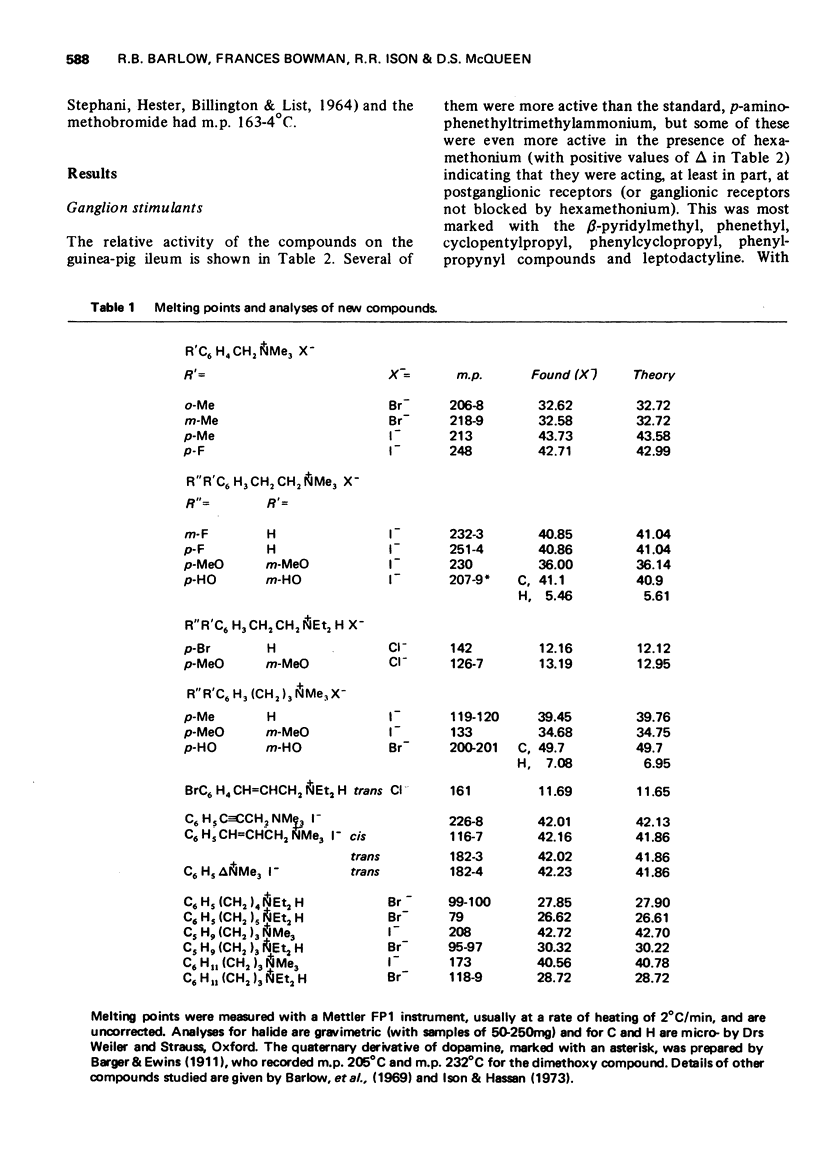
Abstract
1 The guinea-pig isolated ileum has been used to estimate the ability of substituted phenylalkylonium salts (related to nicotine) to stimulate or block receptors in ganglia. The effects of hexamethonium were used to indicate which were the most specific ganglion stimulants; these were tested on the blood-pressure of pithed rats and for neuromuscular blocking activity on the rat diaphragm preparation.
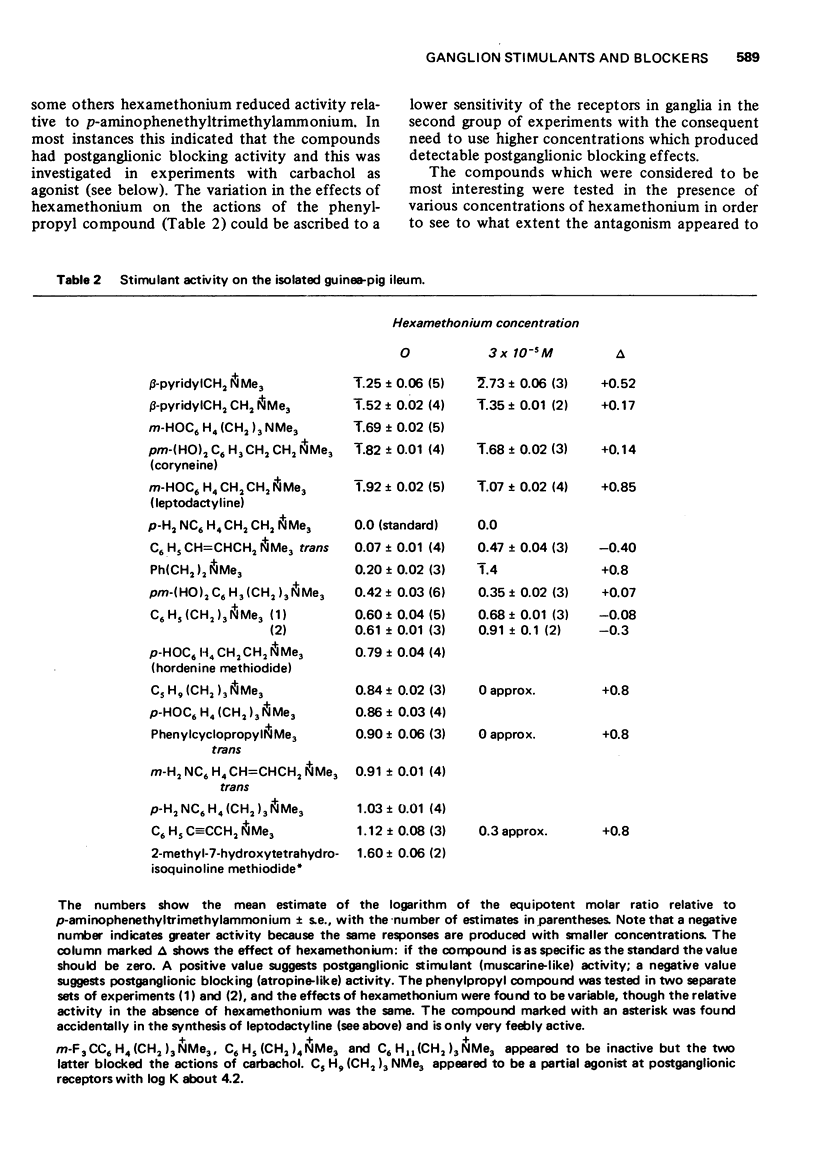
2 m-Hydroxyphenylpropyltrimethylammonium and 3,4-dihydroxyphenethyltrimethylammonium (coryneine, `quaternary dopamine') were the most active and specific ganglion stimulants but their usefulness in vivo may be limited by their neuromuscular blocking activity. The analogous tertiary compounds are being investigated.
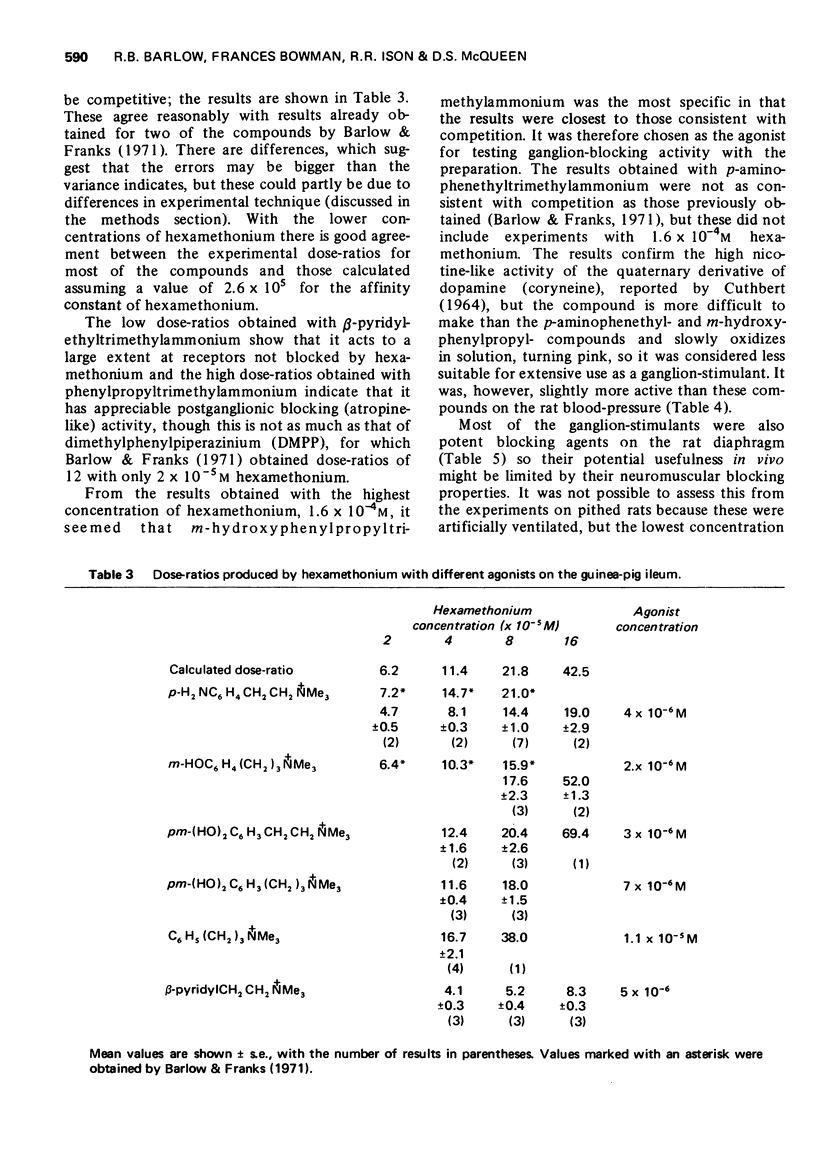
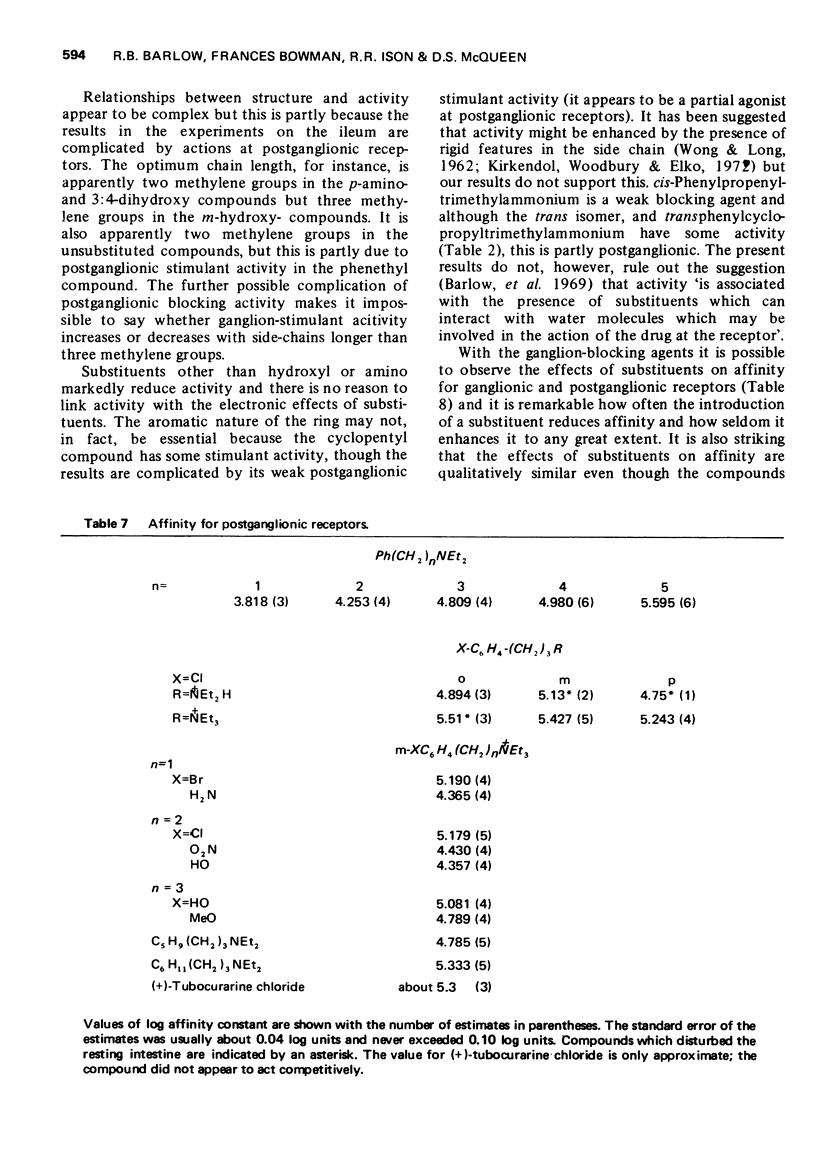
3 The affinities of substances which were blocking agents at ganglionic receptors were measured on the isolated ileum with m-hydroxyphenylpropyltrimethylammonium as agonist. The affinities of selected compounds for postganglionic receptors were measured in experiments on the ileum in the presence of hexamethonium and with carbachol as agonist. Some of the compounds were tested for neuromuscular blocking activity on the rat diaphragm.
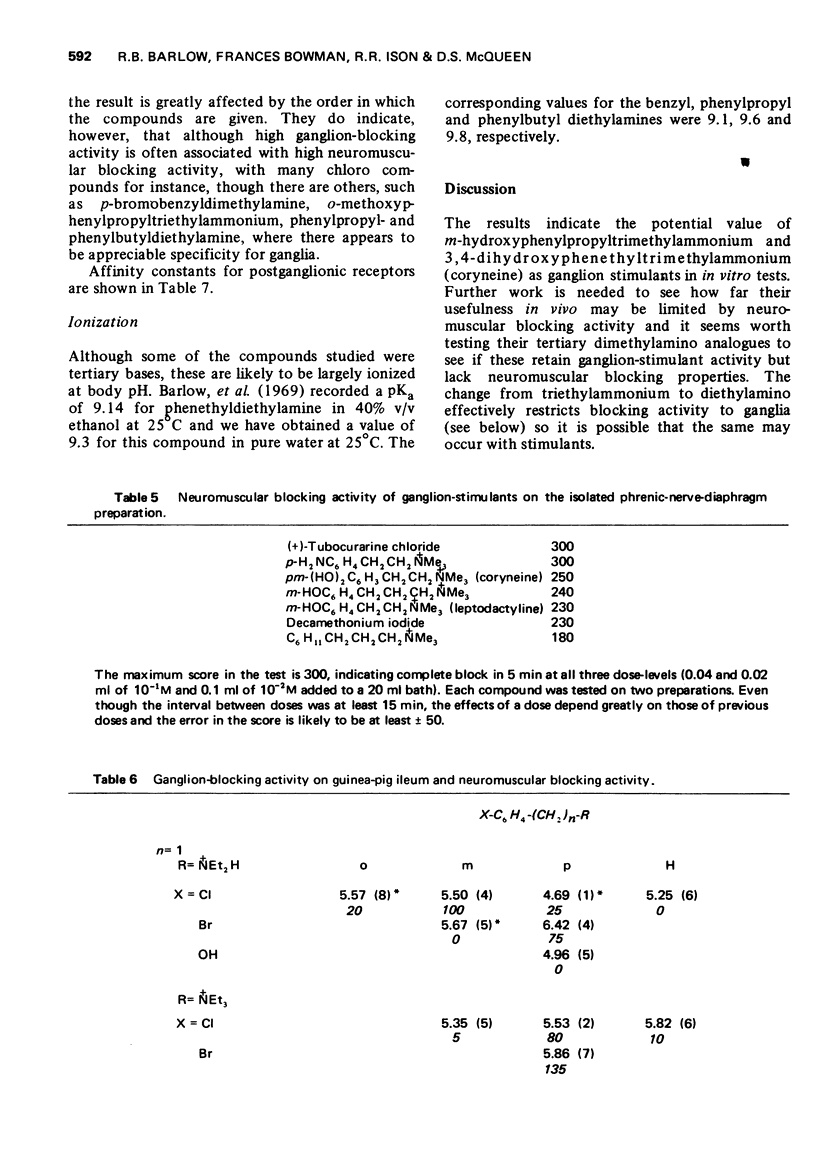
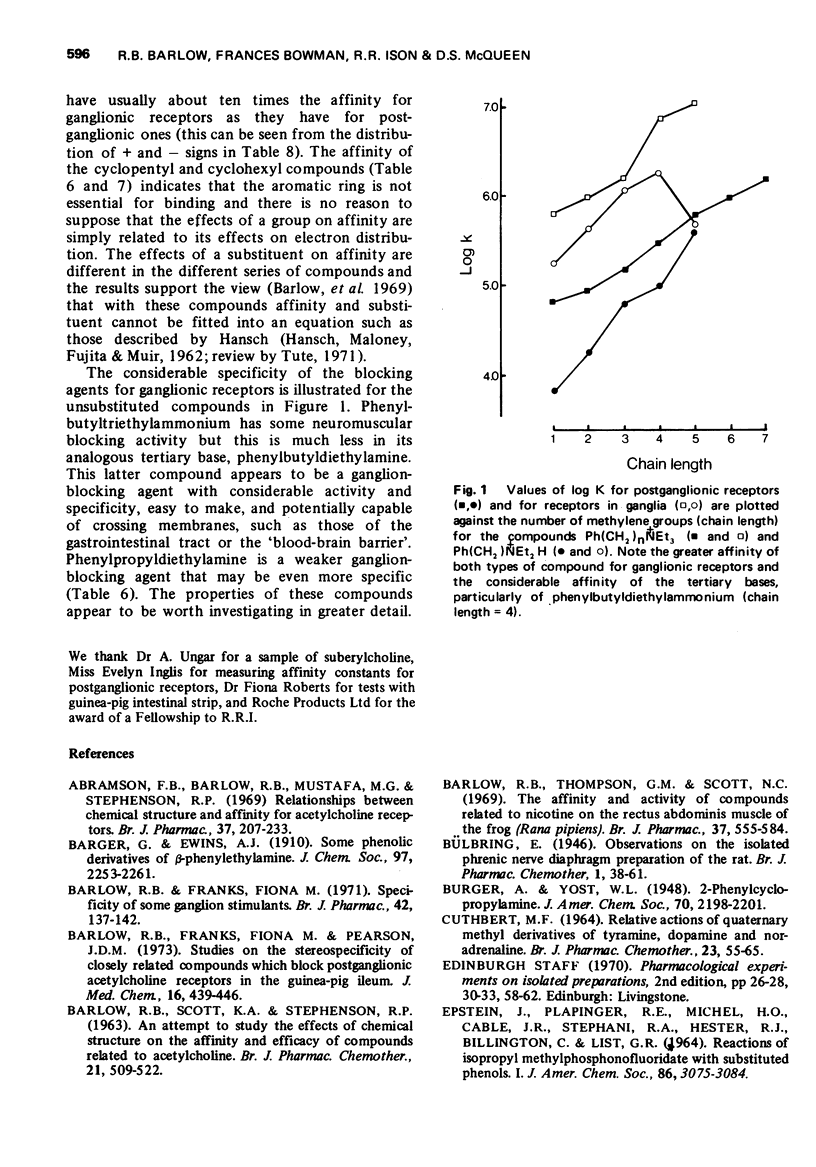
4 Phenylbutyldiethylamine had ganglion-blocking activity greater than pempidine and little postganglionic blocking or neuromuscular blocking activity. Its triethylammonium analogue had higher ganglion-blocking activity but had appreciable neuromuscular blocking activity.
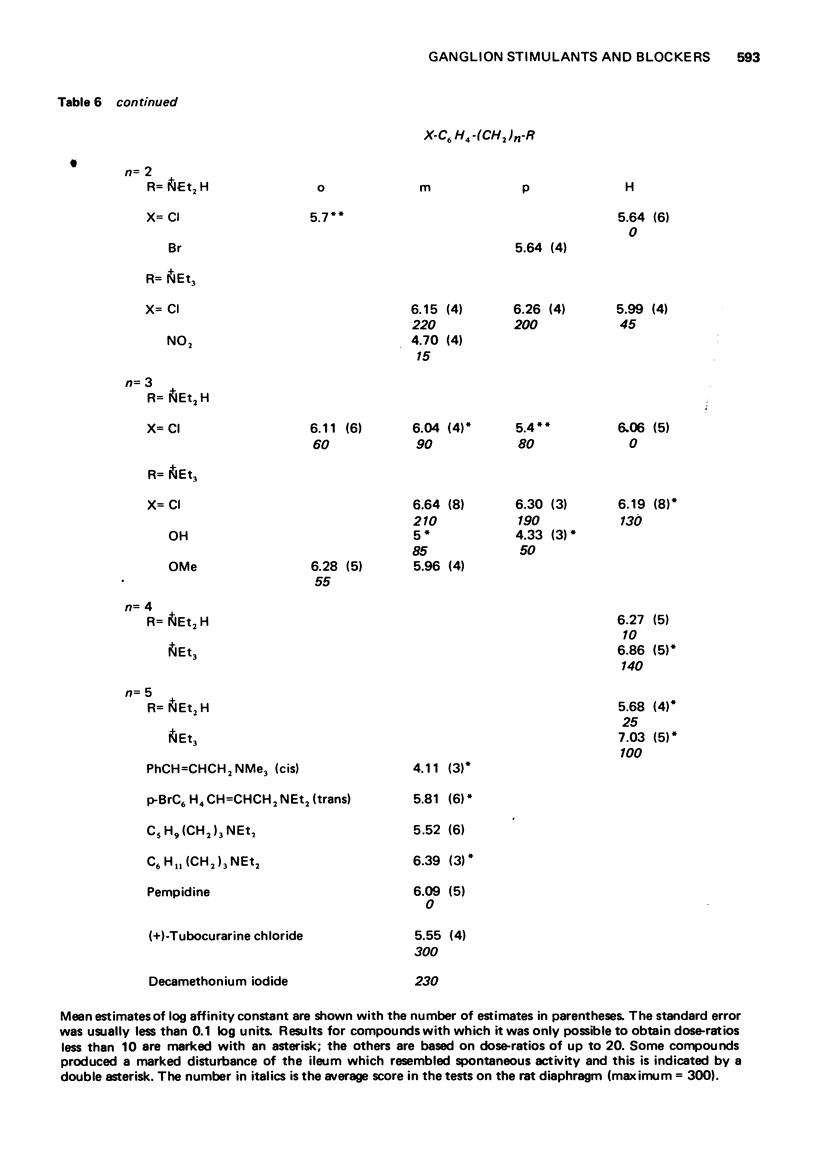
5 The aromatic ring system is not essential either for activity or affinity and the effects of substituents are not related to their effects on electron distribution. Stimulant activity is enhanced only by hydroxyl or amino groups in suitable positions; it is not improved by the presence of rigid features (double or triple bonds or a cyclopropane ring) in the side chain. Affinity is slightly increased by chloro or bromo groups in suitable positions but the unsubstituted compounds are among those with the highest affinity. Substituents have similar effects on affinity for postganglionic receptors, though for these receptors the compounds mostly have only about one-tenth of their affinity for ganglionic receptors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson F. B., Barlow R. B., Mustafa M. G., Stephenson R. P. Relationships between chemical structure and affinity for acetylcholine receptors. Br J Pharmacol. 1969 Sep;37(1):207–233. doi: 10.1111/j.1476-5381.1969.tb09539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW R. B., SCOTT K. A., STEPHENSON R. P. AN ATTEMPT TO STUDY THE EFFECTS OF CHEMICAL STRUCTURE ON THE AFFINITY AND EFFICACY OF COMPOUNDS RELATED TO ACETYLCHOLINE. Br J Pharmacol Chemother. 1963 Dec;21:509–522. doi: 10.1111/j.1476-5381.1963.tb02019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Franks F. M., Pearson J. D. Studies on the stereospecificity of closely related compounds which block postganglionic acetylcholine receptors in the guinea-pig ileum. J Med Chem. 1973 May;16(5):439–446. doi: 10.1021/jm00263a003. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Franks F. Specificity of some ganglion stimulants. Br J Pharmacol. 1971 May;42(1):137–142. doi: 10.1111/j.1476-5381.1971.tb07093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Thompson G. M., Scott N. C. The affinity and activity of compounds related to nicotine on the rectus abdominis muscle of the frog (Rana pipiens). Br J Pharmacol. 1969 Nov;37(3):555–584. doi: 10.1111/j.1476-5381.1969.tb08496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUTHBERT M. F. RELATIVE ACTIONS OF QUATERNARY METHYL DERIVATIVES OF TYRAMINE, DOPAMINE AND NORADRENALINE. Br J Pharmacol Chemother. 1964 Aug;23:55–65. doi: 10.1111/j.1476-5381.1964.tb01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWAI I., HIRAOKA T. STUDIES ON ACETYLENIC COMPOUNDS. XXXV. THE CYCLIZATION REACTION OF SOME PROPARGYLAMMONIUM DERIVATIVES. (1). Chem Pharm Bull (Tokyo) 1963 Dec;11:1564–1568. doi: 10.1248/cpb.11.1564. [DOI] [PubMed] [Google Scholar]
- Ison R. R., Hassan M. M. Clemmensen reduction of Mannich bases: potential ganglionic and nicotinic agents. J Pharm Sci. 1973 Aug;62(8):1388–1390. doi: 10.1002/jps.2600620845. [DOI] [PubMed] [Google Scholar]
- Kiekendol P. L., Woodbury R. A., Elko E. E. Ganglionic activity of 1-(2-bromophenyl)-3-(trimethyl-ammonium)-1-propene bromide (S-11-127). Arch Int Pharmacodyn Ther. 1972 Mar;196(1):25–35. [PubMed] [Google Scholar]
- Tute M. S. Principles and practice of Hansch analysis: a guide to structure-activity correlation for the medicinal chemist. Adv Drug Res. 1971;6:1–77. [PubMed] [Google Scholar]
- WONG K. C., LONG J. P. Nicotinic and muscarinic activity of phenacyl and phenylalkyl trimethylamines. J Pharmacol Exp Ther. 1962 Jul;137:70–75. [PubMed] [Google Scholar]


