Fig. 7.
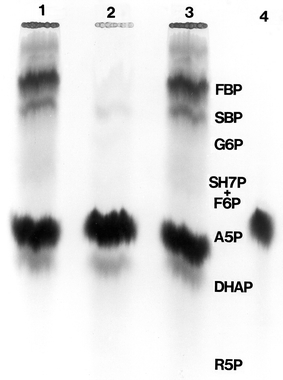
Radioautogram showing the spinach SEP-catalysed conversion of [ U-14C]-Ara 5-P, in the presence of a nine-fold excess of unlabelled Rib 5-P, to other radioactively labelled sugar phosphates. [ U-14C]-Ara 5-P and Rib 5-P were incubated for 1 h with spinach SEP as detailed in Materials and methods. The products of the reaction were separated by descending paper chromatography using the GW3-PBA solvent. Lanes 1 and 3 of the chromatogram show the mixture of 14C-labelled sugar phosphates formed from Ara 5-P dissimilation. Lane 2 shows the radioautograph of products in the absence of Rib 5-P. Lane 4 shows an absence of product formation when boiled SEP was used. The identity, by visualization, of the 14C-labelled sugar phosphates at Lanes 1 and 3 was established by comparison with the positions (not shown in Fig. 7) of the following sugar phosphate marker standards that accompanied the four lanes of the chromatogram, Fru 1, 6-P2 (FBP), Seh 1,7-P2, Seh-7-P, Glc 6-P, Fru 6-P, Ara 5-P, DHAP, Ru 5-P, Xlu 5-P. The one-dimensional chromatography system used does not permit the identification of octulose phosphates (see Kapuscinski et al. 1985 for a list of Rp-i values for some of the above sugar phosphates resolved by the GW3-PBA solvent). The visualization and tentative identification of the labelled products was distinct except for the band running just ahead of Ara 5-P which may have involved ketopentose 5-phosphates and DHAP
