Abstract
Glucose is the principal energy substrate for the the fetus and is essential for normal fetal metabolism and growth. Fetal glucose metabolism is directly dependent on the fetal plasma glucose concentration. Fetal glucose utilization is augmented by insulin produced by the developing fetal pancreas in increasing amounts as gestation proceeds, which enhances glucose utilization among the insulin-sensitive tissues (skeletal muscle, liver, heart, adipose tissue) that increase in mass and thus glucose need during late gestation. Glucose-stimulated insulin secretion increases over gestation. Both insulin secretion and insulin action are affected by prevailing glucose concentrations and the amount and activity of tissue glucose transporters. In cases of intrauterine growth restriction (IUGR), fetal weight-specific tissue glucose uptake rates and glucose transporters are maintained or increased, while synthesis of amino acids into protein and corresponding insulin–IGF signal transduction proteins are decreased. These observations demonstrate the mixed phenotype of the IUGR fetus that includes enhanced glucose utilization capacity, but diminished protein synthesis and growth. Thus, the fetus has considerable capacity to adapt to changes in glucose supply by relatively common and understandable mechanisms that regulate fetal metabolism and growth and could underlie certain later life metabolic disorders such as insulin resistance, obesity and diabetes mellitus.
Other reviews in this edition of The Journal of Physiology by Abigail Fowden and Leslie Myatt present general aspects of placental function and their potential impact on fetal development. This review focuses on glucose, its essential role in fetal metabolism, and recent observations on unique adaptations of fetal metabolism to variations in glucose supply that demonstrate the developmental capacity of the fetus to maintain energy-related metabolic functions in relation to growth. More general discussions of fetal glucose metabolism have been reviewed elsewhere (Hay, 1998, 2003; Hay & Anderson, 2005).
Fetal glucose utilization
Effects of fetal plasma glucose and insulin on fetal glucose metabolism
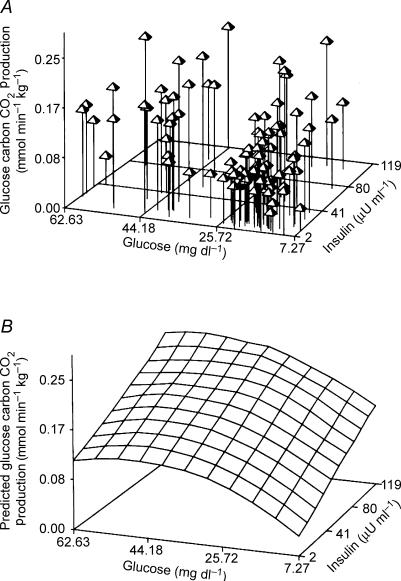
Fetal glucose metabolism depends directly on simultaneous effects of fetal plasma glucose and insulin concentrations, which in experiments in near-term fetal sheep have been shown to act additively to enhance fetal glucose utilization and oxidation to CO2 according to saturation kinetics (Fig. 1) (Hay et al. 1989). The relative proportion of glucose oxidized during short-term 3- to 4-h studies (about 55% in fetal sheep) does not change significantly over the physiological range of fetal glucose utilization rates, indicating little or no effect of glucose or insulin on intracellular pathways of glucose metabolism, at least in the whole fetus; individual tissues and their unique cellular metabolic pathways might vary significantly in their responsiveness to glucose and insulin. Similar observations have been made for fetal metabolic rate, measured as net fetal oxygen uptake rate via the umbilical circulation, which remains relatively constant (±5%) over the entire physiological range of oxygen supply and blood oxygen content, despite marked reductions or increases in glucose supply (Hay et al. 1989; DiGiacomo & Hay, 1990; Aldoretta et al. 1994; McGowan et al. 1995). Acutely, over 2–4 h, preliminary studies in fetal sheep show that carbon from various forms of intracellular glucose appears to maintain oxidation (Davidson & Hay, 2004). Over several days of reduced glucose supply and hypoglycaemia, however, oxidation is maintained by glucose metabolism provided by glycogenolysis and gluconeogenesis and by increasing rates of amino acid oxidation provided by increased protein breakdown (Van Veen et al. 1987). Under these conditions, net fetal protein balance and fetal growth are diminished, as the increased protein breakdown and net use of amino acids for oxidation limits their contribution to net protein synthesis (Carver et al. 1997). In this reciprocal manner, glucose supply and amino acid metabolism are intimately related such that net protein balance and growth are variables that appear subservient to fetal oxidative metabolism. These observations therefore indicate that the fetus develops with mechanisms that tend to keep its energy metabolism relatively constant, while growth is, at times of deficient energy supply, expendable.
Figure 1. Simultaneous effect of fetal plasma glucose and insulin concentrations on fetal glucose oxidation rate.
A, three-dimensional plot of individual values of CO2 production from glucose carbon oxidation at different fetal plasma concentrations of glucose and insulin. B, predicted three-dimensional glucose by insulin surface. Data obtained in near-term fetal sheep (Hay et al. 1989).
Glucose transporters and glucose uptake and metabolism in the fetus
Acute hyperglycaemia-induced increases in plasma insulin concentrations in fetal sheep in late gestation progressively decrease to normal or subnormal values with sustained hyperglycaemia (Carver et al. 1995). Under these chronically hyperglycaemic but normo- to hypoinsulinemic conditions there is a transient increase in brain Glut 1 but not Glut 3 concentration, and a progressive decline in liver and adipose tissue Glut 1 and myocardial and skeletal muscle Glut 1 and Glut 4 concentrations (Das et al. 1999). The chronic hyperglycaemia and reduction in Glut 4 correlate with the development of insulin resistance (Aldoretta & Hay, 2001).
Recent studies in fetal sheep also show a decrease in total fetal glucose uptake and in Glut 4 transporter protein concentration in fetal skeletal muscle after extended periods (2 and 24 h) of high glucose availability (Anderson et al. 2005). Similarly, high fetal insulin concentrations increase Glut 4 protein in fetal skeletal muscle for a limited period, followed by an apparent down-regulation of transporter concentration by 24 h of stimulation. Under such hyperinsulinaemic conditions, however, total fetal glucose uptake remains significantly elevated above control, indicating that insulin signalling mediates the increase in glucose utilization independently of its effect to translocate Glut 4 from intracellular storage pools to the plasma membrane. This possibility is further supported by studies that show increased glucose utilization rate with increasing insulin infusion rates and plasma insulin concentrations despite the lack of further increase in Glut 4 translocation (Anderson et al. 2005).
In contrast, chronic fetal hypoglycaemia (produced by maternal insulin infusion) produces a decline in brain Glut 3, an increase in brain Glut 1, and a subsequent decline in liver Glut 1, but no change in myocardial, skeletal muscle, or adipose tissue Glut 1 or 4 concentrations (Das et al. 1999).
These time-dependent, tissue- and isoform-specific changes in response to altered circulating glucose concentrations indicate that cellular adaptations of fetal glucose transporters produce a reciprocal arrangement that diminishes glucose uptake in the presence of glucose excess and promotes glucose uptake in the presence of glucose insufficiency.
Effects of fetal plasma insulin on signal transduction
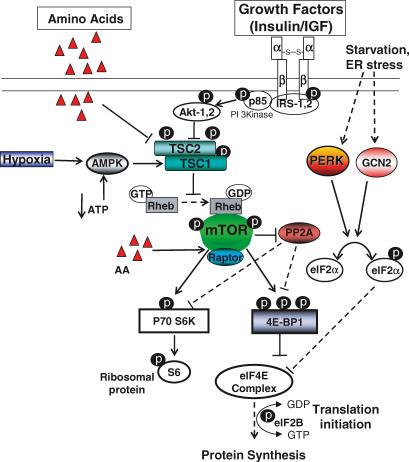
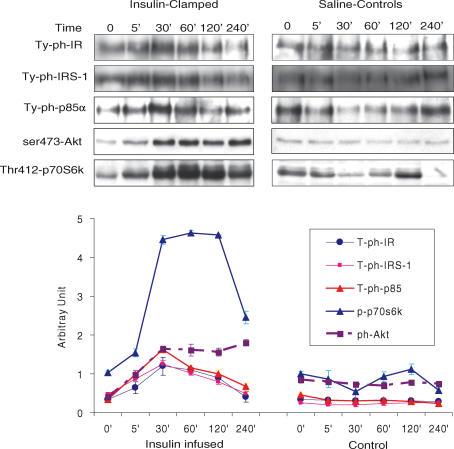
While many previous studies have shown that insulin promotes fetal glucose and amino acid utilization, the mechanisms and time course of insulin action in the fetus have been difficult to determine. Recent serial muscle biopsy studies in anaesthetized fetal sheep have shown a robust increase in the principal insulin signal transduction proteins (IR, IRS-1, p85-PI 3-Kinase, Akt, and p70S6 kinase) with acute insulin stimulation in skeletal muscle during a hyperinsulinaemic–euglycaemic clamp, indicating that the insulin-regulated pathways of glucose uptake and metabolism, initiation of mRNA translation, protein synthesis, and growth are well developed in the late gestation fetal sheep (Fig. 2) (Anderson et al. 2005). These studies also show that the in vivo time course of insulin signal transduction effects is rapid, beginning almost immediately after an abrupt increase in plasma insulin concentration, and variable, with several proteins showing rapid increases in concentration followed by decreases of some and sustained values for others (Fig. 3) (Anderson et al. 2005).
Figure 2. Schematic representation of regulation of translation initiation by amino acids, growth factors and hypoxia.
Insulin, and to a lesser extent the IGFs, activate protein synthesis via a PI3 kinase → Akt → mTOR → p70S6k → 4EBP-1 → eIF4E signal transduction pathway, which can be inhibited at certain points by reduced molecular oxygen supply.
Figure 3. Effect of acute hyperinsulinaemia on signalling protein phosphorylation in fetal skeletal muscle in utero.
The results show a robust insulin response to stimulate IR, IRS-1, p85-PI 3-Kinase, Akt and p70S6 kinase in fetal skeletal muscle in utero during a hyperinsulinaemic–euglycaemic clamp. Repeated muscle biopsies were taken in control (saline-infused) fetuses and in twin fetuses clamped over 240-min period (n = 3 animals/group). From Anderson et al. (2005), with permission.
Fetal insulin secretion
The fetal pancreas develops in the late first to early second trimester, producing measurable insulin concentrations by mid-gestation. There is a gradual increase in basal insulin concentration and glucose- and arginine-induced insulin secretion towards term in fetal sheep (Aldoretta et al. 1998).
Regulation of insulin secretion by glucose concentrations
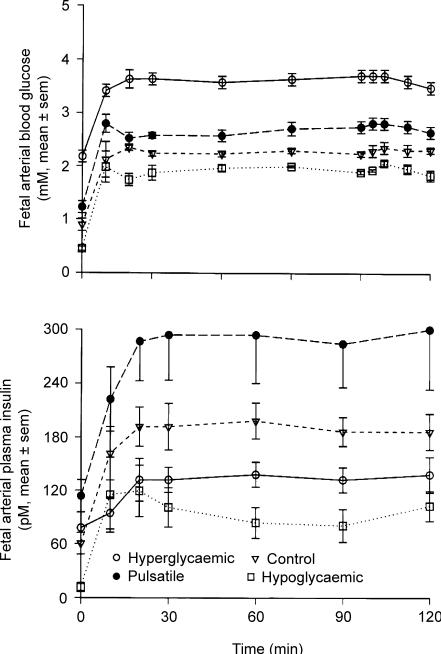
Fetal insulin secretion responds variably to changes in glucose concentration that are dependent on the absolute change in glucose concentration and its duration, magnitude and pattern (Carver et al. 1996). For example, glucose stimulated- and basal insulin secretion in fetal sheep in late gestation are down-regulated in the presence of chronic, sustained, marked hyperglycaemia (Carver et al. 1995; Carver et al. 1996) but pulsatile hyperglycaemia increases fetal insulin secretion, as occurs in human fetuses and neonates (Fig. 4) (Carver et al. 1996). Interestingly, hypoglycaemia also decreases basal and glucose-induced insulin secretion (Limesand & Hay, 2003; Limesand et al. 2005a), evidence of how intrauterine growth restriction, which characteristically among human clinical cases and animal models involves fetal hypoglycaemia, decreases fetal pancreatic development and insulin secretion capacity. Amino acids also regulate insulin secretion, but for the most part, acute and very high physiological to pharmacological changes in a given amino acid's concentration are necessary to elicit a significant change in fetal insulin concentrations (Gresores et al. 1997).
Figure 4. Glucose-stimulated insulin secretion as a function of sustained normal, increased or decreased plasma glucose concentrations in fetal sheep (Hay et al. 1989; Carver et al. 1996).
Four groups of fetal sheep were studied by hyperglycaemic glucose clamp technique after each group had been maintained at a unqiue plasma glucose concentration for 12 days: ▿, control fetuses; ○, fetuses that were markedly and consistently hyperglycaemic (about twice normal); •, fetuses that were mildly hyperglycaemic but had 3 pulses of marked hyperglycaemia lasting 60 min each during a 24-h period; □, hypoglycaemic fetuses that had a plasma glucose about 50% of normal. A, fetal arterial blood glucose concentrations in 4 groups of fetal sheep during a 120 min hyperglycaemic glucose clamp. Values from 30 to 120 min are significantly different (P < 0.01) among all groups. B, fetal arterial plasma insulin concentrations in the same 4 groups of animals during the 120 min hyperglycaemic glucose clamps. Values are means ± s.e.m. From Carver et al. (1996), with permission.
Changes in fetal insulin secretion with intrauterine growth restriction
Human fetuses with severe intrauterine growth restriction (IUGR) have less pancreatic endocrine tissue and exhibit β-cell dysfunction (Van Assche et al. 1977; Nicolini et al. 1990). Such defects, if permanent, might limit β-cell function in later life and contribute to the increased incidence of non-insulin-dependent diabetes mellitus in individuals who were subject to growth restriction in utero. Similar observations have been made experimentally. For example, in the maternal hyperthermia-induced placental insufficiency model of IUGR in sheep, plasma insulin concentrations in the IUGR fetuses are 64% lower at baseline and 77% lower after glucose-stimulated insulin secretion (GSIS) (Limesand et al. 2006). Modelling of changes in plasma insulin concentration in response to glucose and arginine stimulation revealed deficits in the insulin secretion rate in the IUGR fetuses. When tested in vitro, pancreatic islets from the IUGR fetuses secreted insulin in response to increasing glucose and potassium chloride (KCl) concentrations, but the mass of insulin released per IUGR islet was lower than control islets from normally grown fetuses, due to an 82% reduction in their insulin content. A deficiency in islet glucose oxidation, but not total utilization rate, was found at maximal stimulatory glucose concentrations (11 mmol l−1). Interestingly, however, these same studies showed that insulin release as a fraction of total insulin content was greater in the IUGR islets. Thus pancreatic islets from placental insufficiency-induced IUGR fetuses have impaired β-cell stimulus–secretion coupling as a result of reduced glucose-stimulated glucose oxidation rates, insulin biosynthesis and insulin content, despite increased fractional rates of insulin release that results from a greater proportion of releasable insulin in the presence of lower insulin stores (Limesand & Hay, 2003; Limesand et al. 2006).
Additional studies in this model of IUGR have focused on whether apoptosis, decreased replication, or decreased neoformation contributed to the reduced fetal pancreatic β-cell mass (Limesand et al. 2005a). At 90% of term gestation, these studies showed that the IUGR fetal and pancreatic weights were 58 and 59%, respectively, less than pair-fed controls. There also was a selective reduction of pancreatic β-cell mass compared with other pancreatic cell types in the IUGR fetuses. Furthermore, insulin and insulin mRNA contents were less than other pancreatic endocrine hormones in the IUGR pancreases, as were the insulin-positive area (42%) and β-cell mass (76%) in the pancreas. Pancreatic β-cell apoptosis was not different between control and IUGR fetuses, and β-cell capacity for cell cycling, determined by proliferating cell nuclear antigen (PCNA) immunostaining, also was not different between the control and IUGR groups. The percentage of β-cells actually undergoing mitosis, however, was 72% lower in the IUGR fetuses, indicating that reduced nutrient supply to the fetus from placental insufficiency decreased the population of pancreatic β-cells by the end of gestation by lengthening G1, S and G2 stages of interphase and, thereby, decreasing β-cell mitosis.
Based on these results from IUGR fetuses produced by placental insufficiency, the most common cause of human IUGR, it now is important to test whether diminished β-cell mass in IUGR infants at birth is permanent or not, and if not, what nutrient and/or hormonal factors might best promote improved β-cell growth and development. It also is important to determine whether such diminished β-cell mass in IUGR fetuses, if permanent or at least not adequately compensated for after birth, might contribute to insufficient insulin production in later life and, thus, a predisposition to non-insulin-dependent diabetes.
Changes in fetal insulin secretion with hypoglycaemia
A common biochemical characteristic of fetuses with IUGR is relative hypoglycaemia; it is reasonable therefore to consider whether fetal pancreatic adaptations to hypoglycaemia alone, without other general nutrient deficiencies associated with IUGR (reduced amino acid and oxygen delivery, for example), would, by itself, limit the capacity of fetal pancreatic β-cells to produce and/or secrete insulin. Preliminary studies measured glucose-stimulated insulin secretion (GSIS) in normal control fetal sheep, fetal sheep made hypoglycaemic by maternal insulin infusion for 2 weeks in late gestation, and in a similar hypoglycaemic group of fetuses after a 5-day euglycaemic recovery period (Limesand & Hay, 2003). Hypoglycaemia significantly decreased plasma insulin concentrations in the hypoglycaemic (0.13 ± 0.01 ng ml−1) and recovery fetuses (0.11 ± 0.01 ng ml−1); insulin concentrations returned to euglycaemic control values (0.30 ± 0.01 ng ml−1) in recovery fetuses (0.29 ± 0.04 ng ml−1) during their euglycaemic recovery period. Mean steady state plasma insulin concentration during the GSIS study was reduced in hypoglycaemic fetuses (0.40 ± 0.07 versus 0.92 ± 0.10 ng ml−1 in controls); there was some recovery in the recovery group of fetuses (0.73 ± 0.10 ng ml−1), but their mean insulin concentration during the GSIS period was still less than in the control group. Non-linear modelling of GSIS showed that the time from onset of hyperglycaemia until insulin concentration reached 50% of its final GSIS value (insulin secretion response time) was greater (P < 0.01) in both hypoglycaemic (15.6 ± 2.8 min) and recovery fetuses (15.4 ± 1.5 min) than in control fetuses (6.3 ± 1.1 min). Insulin secretion responsiveness to arginine also was reduced by hypoglycaemia (0.98 ± 0.11 ng ml−1 hypoglycaemic versus 1.82 ± 0.17 ng ml−1 controls, P < 0.05) and did not recover with normalization of glucose concentration (1.21 ± 0.15 ng ml−1 recovery, P < 0.05 versus control). A further study of islets from hypoglycaemic fetuses showed increased islet insulin concentration without an increase in the number of islets per pancreatic wet weight or the number of β-cells per islet (S. Limesand & W. W. Hay Jr, unpublished data).
In contrast to the IUGR fetuses from generalized placental insufficiency, therefore, late gestation chronic hypoglycaemia did not develop a failure of β-cell growth or replication, but instead developed a defect in insulin secretion. This was independent of the capacity of these islets to take up and oxidize glucose, indicating that the defect in insulin secretion was likely to be downstream of early phases of glucose–insulin secretion coupling (Rozance et al. 2003a,b, 2004). These studies showed therefore that hypoglycaemia itself could reduce fetal pancreatic insulin secretion responsiveness and that this inhibition, while not proven to be permanent, still showed delayed recovery, indicating a possible mechanism to account for a predisposition to Type 2 diabetes that could begin in fetal life.
Other aspects of glucose and amino acid metabolism in IUGR
Up-regulation of mechanisms regulating glucose utilization
Many different models of IUGR (Wallace et al. 2002) have shown that when the fetus is deprived of glucose, either selectively such as with maternal hypoglycaemia or generally as with placental insufficiency, fetal weight-specific glucose utilization rate is not very much different from normal rates (Wallace et al. 2005). These conditions both produce fetal hypoglycaemia, which increases the maternal–fetal glucose concentration gradient and thus the driving force for glucose transport across the placenta, thereby compensating in part for the reduction in glucose supply. For the fetus to accomplish this, there must be an increase in the fetal tissue capacity for glucose uptake and/or utilization, which could come about by increased concentrations and/or activity and/or plasma membrane localization of glucose transporters, increased insulin signal transduction and thereby effectiveness to promote Glut 4 (and perhaps Glut 1) translocation to the cell membrane, or mechanisms of insulin metabolism into oxidative and/or non-oxidative pathways. In both fetal sheep and fetal rats with IUGR, Glut 1 and Glut 4 concentrations in myocardium, adipose tissue and skeletal muscle do not decrease with sustained hypoglycaemia (Das et al. 1999; Sadiq et al. 1998, 1999), perhaps a positive adaptation to maintain glucose utilization despite hypoglycaemia.
While it has not been determined if the maintenance of glucose transporter concentrations is sufficient by itself to maintain fetal glucose utilization at normal rates, recent preliminary studies have tested how well fetal sheep with placental insufficiency and IUGR could metabolize glucose at low plasma insulin concentrations (Limesand et al. 2005b). Fetal glucose metabolism was determined under basal and hyperglycaemic conditions in near-term IUGR fetal sheep, generated by exposing pregnant ewes to elevated ambient temperatures during the middle third of gestation, and pair-fed control fetuses. Relative amounts of Glut 1 per total protein were not different between control and IUGR fetuses for liver or skeletal muscle, but Glut 1 was greater in the brain of the IUGR fetus. Glut 4 levels also were not different between treatments in fetal skeletal muscle. Plasma glucose clearance rate, calculated as glucose utilization rate divided by the arterial plasma glucose concentration, was not different between the IUGR and the control fetuses in the basal period, even though plasma glucose and insulin concentrations were significantly lower in the IUGR fetuses. Predicted glucose utilization rates at the studied glucose and insulin concentrations, derived from previous measurements in normal fetal sheep of the same gestational age, were significantly reduced from those actually found in the IUGR fetuses, averaging 54% lower under basal and 81% lower under hyperglycaemic conditions. Other preliminary data (M. A. Anderson, J. Friedman, W. W. Hay Jr, unpublished results) in these placental insufficiency-induced IUGR fetal sheep have shown increased levels of insulin receptor protein and decreased levels of the insulin signal transduction inhibitors, P85 protein subunit of phosphatidylinositol 3 kinase (PI3K), which negatively regulates the effect of insulin to promote Glut 4 translocation from inactive intracellular storage pools to active sites in the cell membrane where it enhances glucose uptake across the cell membrane, and glycogen synthase kinase (GSK), which negatively regulates glycogen synthase and the synthesis of glucose into glycogen. Therefore, fetal tissues in these chronically IUGR fetuses had adapted to their hypoglycaemic environment by developing mechanisms to promote glucose uptake and utilization, possibly via enhanced insulin action.
Down-regulation of mechanisms regulating growth
The principal characteristic of chronically IUGR fetuses is a slower rate of growth. Perhaps such a slower rate of growth allows normal cellular metabolism to continue when placental insufficiency deprives the fetus of normal rates of nutrient substrates for both metabolism and growth. Regardless of this teleological hypothesis, data in fetal sheep regarding mechanisms that regulate the synthesis of amino acids into protein show the opposite of up-regulated glucose utilization capacity. Instead, data for amino acid incorporation into protein have shown decreased insulin signal transduction proteins, including mammalian target of rapamycin (mTOR), p70S6K, and eukaryotic initiation factor 4E (eIF4E) that regulate the synthesis of amino acids into protein, plus an increase in the binding protein 4E-BP1 that would inhibit the function of eIF4E. Furthermore, after first showing that insulin selectively activates the mitogen-activated protein kinase (MAPK) pathway in skeletal muscle and liver (Stephens et al. 2001), more recent studies have found reduced skeletal muscle cell ERK-2 levels in IUGR fetuses (J. Friedman, T. R. H. Regnault & W. W. Hay Jr, unpublished data). These proteins regulate cell turnover and thus hyperplasia; thus, a decrease in their levels would indicate inhibition of cell growth. These recent observations provide support for the hypothesis that the effect of insulin to promote cell turnover and hyperplasia is reduced at several regulatory steps in chronically IUGR fetuses from placental insufficiency.
Other changes in fetal metabolism in response to glucose (and thus insulin) deficiency have been noted, including glucose production from glycogen breakdown and the substitution of amino acids for glucose oxidation with acute glucose deprivation. With chronic glucose deprivation, however, fetal gluconeogenesis develops (Narkewicz et al. 1993) and protein breakdown diminishes, allowing conservation of amino acids in protein structure while growth remains limited by the decrease in amino acid transfer to the fetus by the placenta (van Veen et al. 1987; Carver et al. 1997). If such adapatations are linked to the decreased insulin signal transduction protein concentrations found in skeletal muscle and liver in IUGR fetuses, it is possible that chronic nutrient deprivation leading to growth restriction might portend limited capacity for protein growth in later life.
Conclusions and future studies
The fetus has considerable capacity to adapt metabolically to acute and chronic changes in glucose supply by relatively common and understandable mechanisms. Current research is designed to test whether these molecular and physiological changes in glucose utilization capacity could be reversed by selective re-introduction of nutrient (glucose or amino acids, or both) and hormonal (insulin or IGF-1 or both) supplies, directly by infusion into the fetus or indirectly by infusion into the mother. Future studies should find out whether such adaptations might lead to long-term, possibly permanent changes in metabolic capacity that could underlie certain childhood and adult metabolic disorders such as insulin resistance, obesity and diabetes mellitus. An additional goal is to re-examine the mechanisms by which protein supplements to pregnant women often leads, surprisingly, to increased fetal morbidity and even mortality.
Acknowledgments
This work was supported by grants HD20761 and DK35836 from the National Institutes of Health.
References
- Aldoretta PW, Carver TD, Hay WW., Jr Ovine uteroplacental glucose and oxygen metabolism in relation to chronic changes in maternal and fetal glucose concentrations. Placenta. 1994;15:753–764. doi: 10.1016/0143-4004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Aldoretta PW, Carver TD, Hay WW., Jr Maturation of glucose-stimulated insulin secretion in fetal sheep. Biol Neonate. 1998;73:375–386. doi: 10.1159/000014000. [DOI] [PubMed] [Google Scholar]
- Aldoretta PW, Hay WW., Jr Chronic hyperglycemia induces insulin resistance and glucose intolerance in fetal sheep. Pediatr Res. 2001;49:307A. abstract 1758. [Google Scholar]
- Anderson MS, Thamotharan M, Kao D, Devaskar SU, Qiao L, Friedman JE, Hay WW., Jr Effects of acute hyperinsulinemia on insulin signal transduction and glucose transporters in ovine fetal skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R473–R481. doi: 10.1152/ajpregu.00405.2004. [DOI] [PubMed] [Google Scholar]
- Carver TD, Anderson SM, Aldoretta PA, Esler AL, Hay WW., Jr Glucose suppression of insulin secretion in chronically hyperglycemic fetal sheep. Pediatr Res. 1995;38:754–762. doi: 10.1203/00006450-199511000-00020. [DOI] [PubMed] [Google Scholar]
- Carver TD, Anderson SM, Aldoretta PW, Hay WW., Jr Effect of low-level basal plus marked ‘pulsatile’ hyperglycemia on insulin secretion in fetal sheep. Am J Physiol. 1996;271:E865–E871. doi: 10.1152/ajpendo.1996.271.5.E865. [DOI] [PubMed] [Google Scholar]
- Carver TD, Quick AA, Teng CC, Pike AW, Fennessey PV, Hay WW., Jr Leucine metabolism in chronically hypoglycemic hypoinsulinemic growth-restricted fetal sheep. Am J Physiol. 1997;272:E107–E117. doi: 10.1152/ajpendo.1997.272.1.E107. [DOI] [PubMed] [Google Scholar]
- Das UG, Schroeder RE, Hay WW, Jr, Devaskar SU. Time-dependent and tissue-specific effects of circulating glucose on fetal ovine glucose transporters. Am J Physiol. 1999;276:R809–R817. doi: 10.1152/ajpregu.1999.276.3.R809. [DOI] [PubMed] [Google Scholar]
- Davidson LS, Hay WW., Jr Effect of hyperinsulinemia on amino acid utilization independent of glucose metabolism in the ovine fetus. Pediatr Res. 2004;55:382A. doi: 10.1152/ajpendo.00028.2006. abstract. [DOI] [PubMed] [Google Scholar]
- Digiacomo JE, Hay WW., Jr Fetal glucose metabolism and oxygen consumption during sustained hypoglycemia. Metabolism. 1990;39:193–202. doi: 10.1016/0026-0495(90)90075-n. [DOI] [PubMed] [Google Scholar]
- Gresores A, Anderson S, Hood D, Zerbe GO, Hay WW., Jr Separate and joint effects of arginine and glucose on ovine fetal insulin secretion. Am J Physiol. 1997;272:E68–E73. doi: 10.1152/ajpendo.1997.272.1.E68. [DOI] [PubMed] [Google Scholar]
- Hay WW., Jr . Glucose metabolism in the fetal-placental unit. In: Cowett RM, editor. Principles of Perinatal-Neonatal Metabolism. New York: Springer-Verlag; 1998. pp. 337–368. [Google Scholar]
- Hay WW., Jr . Nutrition and development of the fetus: carbohydrate and lipid metabolism. In: Walker WA, Watkins JB, Duggan CP, editors. Nutrition in Pediatrics (Basic Science and Clinical Applications) Ontario: BC Decker Inc.; 2003. pp. 449–470. [Google Scholar]
- Hay WW, Jr, Anderson MS. Fuel homeostasis in the fetus and neonate. In: Jameson JL, Degroot LJ, editors. Endocrinology. Philadelphia: W.B. Saunders Company; 2005. pp. 3387–3406. [Google Scholar]
- Hay WW, Jr, Digiacomo JE, Meznarich HK, Hirst K, Zerbe G. Effects of glucose and insulin on fetal glucose oxidation and oxygen consumption. Am J Physiol. 1989;256:E704–E713. doi: 10.1152/ajpendo.1989.256.6.E704. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Hay WW., Jr Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol. 2003;547:95–105. doi: 10.1113/jphysiol.2002.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005a;288:R1297–R1305. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., JR Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 2006 doi: 10.1210/en.2005-0900. in press. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Smith D, Rozance PJ, Hay WW., Jr Enhanced Insulin sensitivity in fetal sheep with placental insufficiency and intrauterine growth restriction (IUGR)3rd International Congress of the Developmental Origins of Health and Disease, Toronto, Ontario, Canada, November 16–20, 2005. Pediatr Res. 2005b;58:1053. abstract p1-049. [Google Scholar]
- McGowan JE, Aldoretta PW, Hay WW., Jr Contribution of fructose and lactate produced in placenta to calculation of fetal glucose oxidation rate. Am J Physiol. 1995;269:E834–E839. doi: 10.1152/ajpendo.1995.269.5.E834. [DOI] [PubMed] [Google Scholar]
- Narkewicz MR, Carver TD, Hay WW., Jr Induction of cytosolic phosphoenolpyruvate carboxykinase in the ovine fetal liver by chronic fetal hypoglycemia and hypoinsulinemia. Pediatr Res. 1993;33:493–496. doi: 10.1203/00006450-199305000-00014. [DOI] [PubMed] [Google Scholar]
- Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22:426–430. doi: 10.1055/s-2007-1004939. [DOI] [PubMed] [Google Scholar]
- Rozance PJ, Limesand SW, Wyckoff MH, Hay WW., Jr Correlation between insulin secretion and glucose metabolism in ovine fetal pancratic islets. Pediatric Res. 2003a;53:394A. abstract 2233. [Google Scholar]
- Rozance PJ, Limesand SW, Wyckoff MH, Hay WW., Jr Diminished insulin secretion is not associated with changes in glucose utilization or oxidation in pancreatic islets obtained from hypoglycemic fetal sheep. Endocrine Societies 85th Annual Meeting, Philadelphia, PA. 2003b abstract p1-410. [Google Scholar]
- Rozance PJ, Limesand SW, Wyckoff MH, Hay WW., Jr Chronic hypoglycemia reduces potassium and calcium stimulated insulin secretion in pancreatic islets obtained from fetal sheep. Pediatric Res. 2004;55:382A. abstract. [Google Scholar]
- Sadiq HF, Das UG, Tracy TF, Devaskar SU. Intra-uterine growth restriction differentially regulates perinatal brain and skeletal muscle glucose transporters. Brain Res. 1999;823:96–103. doi: 10.1016/s0006-8993(99)01145-2. [DOI] [PubMed] [Google Scholar]
- Sadiq HF, deMello DE, Devaskar SU. The effect of intrauterine growth restriction upon fetal and postnatal hepatic glucose transporter and glucokinase proteins. Pediatr Res. 1998;43:91–100. doi: 10.1203/00006450-199801000-00014. [DOI] [PubMed] [Google Scholar]
- Stephens E, Thureen PJ, Goalstone ML, Anderson MS, Leitner JW, Hay WW, Jr, Draznin B. Fetal hyperinsulinemia increases farnesylation of p21 Ras in fetal tissues. Am J Physiol Endocrinol Metab. 2001;281:E217–E223. doi: 10.1152/ajpendo.2001.281.2.E217. [DOI] [PubMed] [Google Scholar]
- Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. 1977;84:751–753. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- Van Veen LC, Teng C, Hay WW, Jr, Meschia G, Battaglia FC. Leucine disposal and oxidation rates in the fetal lamb. Metabolism. 1987;36:48–53. doi: 10.1016/0026-0495(87)90062-x. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Aitken RP, Milne JS. Nutritionally-mediated placental growth restriction in the growing adolescent: consequences for the fetus. Biol Reprod. 2005 doi: 10.1095/biolreprod.104.030965. DOI 10.1095/biolreprod.104.030965. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Leitch N, Hay WW., Jr Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1027–R1036. doi: 10.1152/ajpregu.00465.2001. [DOI] [PubMed] [Google Scholar]