Fig. 1.
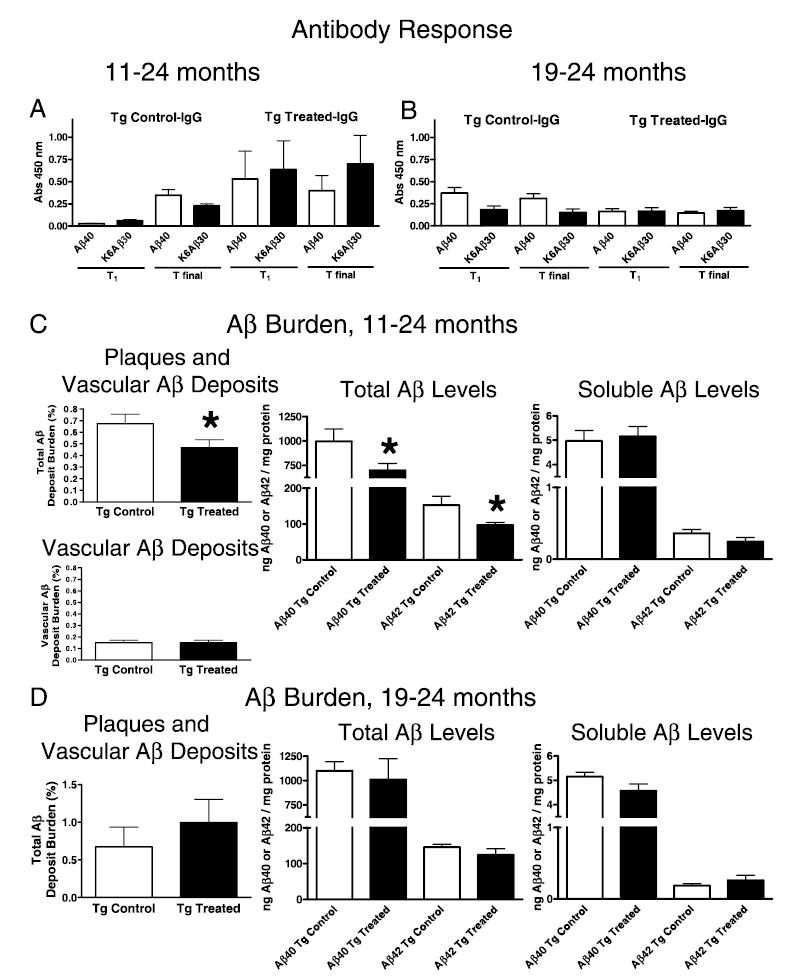
Antibody levels and brain amyloid burden. Groups were treated from 11 to 24 months (A and C) and from 19 to 24 months (B and D). (A) 11–24 months. K6Aβ1–30 in alum adjuvant elicits a good antibody response that is maintained (T1, 13 months; Tfinal, 24 months). The antibodies recognize both the immunogen and Aβ1–40. Vehicle-treated controls have some autoantibodies at old age. Treatment and antibody type measured (IgG) is indicated in the title. The x-axis depicts which peptide (coated on ELISA plate) the antibodies are recognizing. The y-axis depicts the absorbance at 450 nm. (B) 19–24 months. K6Aβ1–30 in alum adjuvant is not immunogenic when treatment is started at old age (19 months). (C) 11–24 months. Cortical amyloid burden (parenchymal and vascular) was reduced by 31% (P < 0.05) in Tg2576 mice immunized with K6Aβ1–30 in alum adjuvant from 11 to 24 months of age, compared to control mice. When analysed separately, vascular amyloid burden was not significantly altered by the immunotherapy. Total brain Aβ levels (Aβ1–40, 30% reduction, P = 0.03; Aβ1–42, 37% reduction, P = 0.02) were reduced to a similar extent as total amyloid burden. Levels of soluble Aβ were not significantly affected although soluble Aβ1–42 was reduced by 32% in the immunized mice (P = 0.08). (D) 19–24 months. No significant difference in cortical amyloid burden was observed between the immunized and nonimmunized Tg mice (P = 0.206). The error bars are standard error of the mean that applies also to all subsequent figures.
