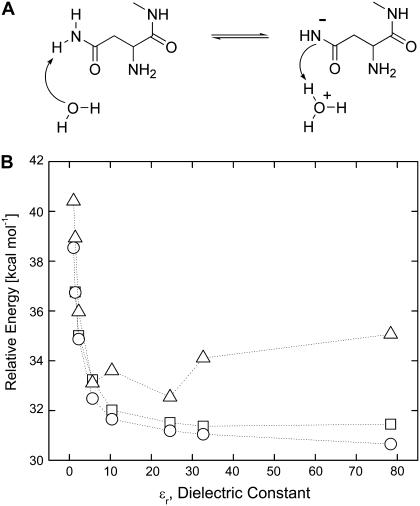
FIGURE 5.
Energy of ionization of asparagine side-chain nitrogen (panel A) calculated in solvents of different dielectric constants: vacuum (ɛr = 1), argon (ɛr = 1.43), benzene (ɛr = 2.247), chlorobenzene (ɛr = 5.621), dichloroethane (ɛr = 10.36), ethanol (ɛr = 24.55), methanol (ɛr = 32.63), and water (ɛr = 78.39). Each implicit solvent has unique parameters such as radius and density. Gibbs energy is shown by blue triangles. Note that the peptide nitrogen has only one proton in this case. Gas phase coordinates, energy in solvent (○); solvent optimized coordinates, energy (□); and Gibbs free energy (Δ).