Figure 6-6921.
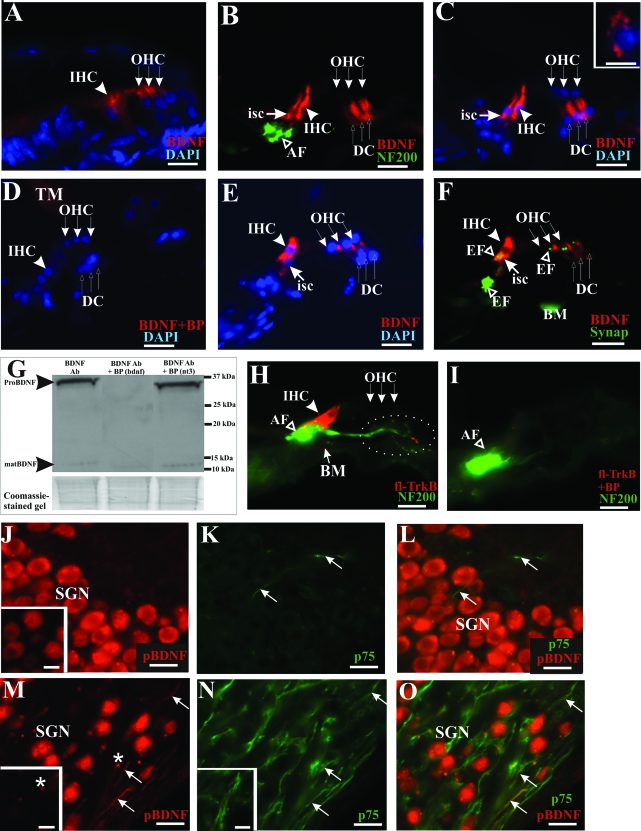
BDNF protein is expressed in both hair and supporting cells of the adult organ of Corti as well as spiral ganglion neurons. Both inner and outer hair cells from the postnatal rat cochlea expressed BDNF, as shown in A (arrowhead pointing to inner hair cell and row of three arrows to outer hair cells). In contrast, in the adult rat cochlea, BDNF expression was localized to inner supporting cells (B and C, isc), inner hair cells (B and C, bold arrowhead, IHC), and Deiters’ cells (B and C, row of three upward-pointing arrows, DC). Afferent innervation was identified using NF200 antibody (B, open arrowhead, AF), and specific cells of the organ of Corti were recognized using DAPI nuclear staining (C). D: The BDNF staining pattern was completely abolished on pre-incubation of the antibody with the recommended antigenic peptide. As a positive control, we also observed BDNF immunoreactivity in cerebellar granule neuron (C, inset). Expression pattern of BDNF in the adult organ of Corti was also verified in mice where immunolabeling was detected in inner hair cells (E and F, bold arrowhead, IHC), inner supporting cell (E and F, arrow, isc), Deiters’ cells (E and F, row of three upward-pointing arrows, DC). Consistently, no BDNF immunoreactivity could be found in the outer hair cells of adult rat (B and C, row of three downward-pointing arrows) or mouse (E and F, row of three downward-pointing arrows). BDNF immunoreactivity was also localized opposite synaptophysin-labeled efferent nerve endings (F, open arrowhead, EF), whereas labeling at the basilar membrane is nonspecific (F, BM). G: The specificity of the BDNF antibody was further investigated in Western blot analysis of cochlear proteins where both a ∼34-kd fragment corresponding to the pro-neurotrophin form and a ∼13-kd fragment representing the mature neurotrophin (left lane) were detected. These bands could not be detected when the antibody was pre-incubated with its antigenic peptide (middle lane). Pre-incubating the antibody with an irrelevant antigenic peptide derived from neurotrophin-3 did not result in a loss of these bands (right lane). A Coomassie-stained gel below indicated analysis of comparable amounts of proteins in this experiment. H: Full-length TrkB immunoreactivity was detected primarily at the baso-lateral region of inner hair cells (bold arrowhead, IHC) and partially colocalized with NF200 immunostaining at the afferent nerve endings (open arrowhead, AF). I: TrkB immunolabeling was abolished in neutralization experiment with the relevant antigenic peptide, without affecting NF200 immunostaining. J: SGNs from a normal hearing rat cochlea expressed BDNF protein in their soma, as shown using an antibody recognizing the uncleaved pro-neurotrophin form of BDNF (pBDNF) and another antibody targeting the mature BDNF isoform (inset). The p75NTR immunoreactivity could be weakly detected in projection fibers (K, arrows) in a normal hearing rat cochlea, and no colocalization could be observed between p75NTR and pBDNF in these fibers (L, arrows). However, in an aminoglycoside-deafened cochlea, pBDNF immunoreactivity appeared strongly in the neural fibers of the Rosenthal’s canal of deafened animals (M, arrows), shown here after 3 months of deafness. These pBDNF-positive fibers also expressed p75NTR (N, arrows) and showed colocalization with a subset of p75NTR-positive fibers (O, arrows). Pre-incubating the pBDNF antibody with its antigenic peptide abolished the filamentous fluorescence signal in these fibers (M, inset), whereas p75NTR immunoreactivity was not affected (N, inset). M: Nonspecific staining, presumably representing cellular debris, could be observed as fluorescent spots (asterisk) that could not be blocked (inset), suggesting that these fluorescent spots did not reflect pBDNF immunoreactivity. Cochlear sections shown here were taken from the middle turn. Scale bars: 20 μm; except inset in C (10 μm). Original magnification: ×40; except inset in C (×100).