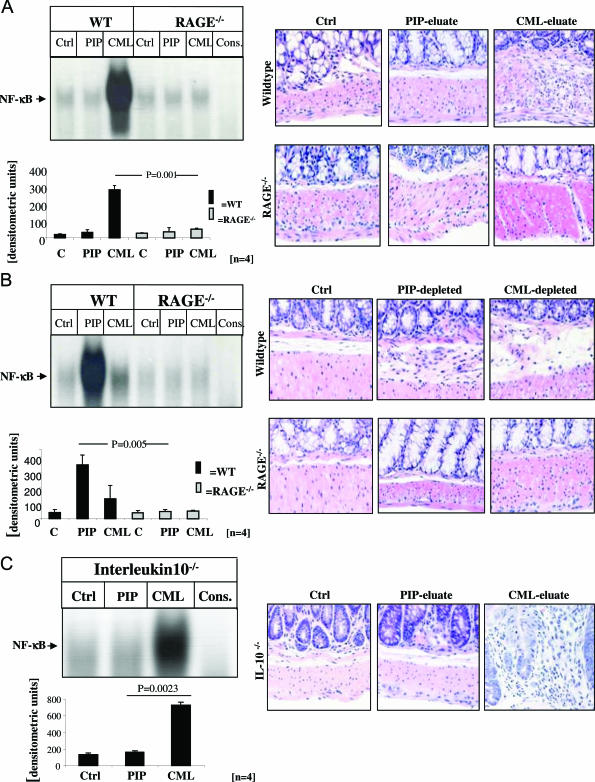
FIGURE 8.
CML-mps derived from highly inflamed CD gut cause prominent activation of NF-κB in the gut from WT and IL-10−/− mice. A and B: Left panels: Protein extracts (15 mg) from highly inflamed CD gut from four representative CD patients were incubated with Sepharose-immobilized antibodies to either PIP kinase or CML-mps and adsorbed proteins were eluted as above. Seventy-five μg of the resulting eluates (A, C) and 75 μg of the resulting supernatants (comprising the solution from which proteins had been eluted) (B) were rectally applied into the colon of WT (A and B, lanes 2 and 3), RAGE−/− (A and B, lanes 5 and 6) mice, and of IL-10−/− mice (C, lanes 2 and 3) at days 0, 2, 4, and 6. Untreated gut extracts from WT (A and B, lane 1), RAGE−/− mice (A and B, lane 4), and IL-10−/− mice (C, lane 1) served as an additional control. Specificity of NF-κB binding (using the same sample as in lane 7 in A and B and lane 4 in C) was shown by including a 160-fold molar excess of unlabeled consensus NF-κB oligonucleotide (A and B, lane 7). At day 7, mice were sacrificed and gut tissue was collected for EMSA. Nuclear extracts were prepared and assayed for NF-κB binding activity as shown for representative mice (A–C: top left), and the bar graphs summarize the densitometric analysis of all experiments (A–C: bottom left). The corresponding representative immunohistochemical analyses are shown on the right.