Abstract
17β-Estradiol (E2) accelerates reendothelialization and increases the number of circulating endothelial progenitor cells (EPCs), but whether fibroblast growth factor-2 (FGF2) is involved in these processes remains unknown. Here we explored the role of FGF2 in the effect of E2 on reendothelialization and EPC levels in a mouse model. As previously reported, E2 increased both the velocity of reendothelialization and the number of circulating EPCs in ovariectomized wild-type (Fgf2+/+) mice. In contrast, the effect of E2 on both parameters was abolished in FGF2-deficient mice (Fgf2−/−), demonstrating that FGF2 is absolutely required for these effects of E2. To test the implication of medullary and extramedullary FGF2, we developed chimeric mice by grafting Fgf2−/− bone marrow to Fgf2+/+ [Fgf2−/− bone marrow (BM) = >Fgf2+/+] mice and observed that the effect of E2 on both reendothelialization and EPC levels was abolished. In contrast, both effects of E2 in Fgf2+/+BM = >Fgf2−/− mice were similar to those observed in Fgf2+/+ mice, demonstrating that only BM-derived, but not extramedullary, FGF2 is required for both effects. Interestingly, E2 was found to markedly increase both FGF2lmw and FGF2hmw in bone marrow. In conclusion, FGF2, specifically medullary FGF2, is necessary and sufficient to mediate the accelerative effect of E2 on both reendothelialization and EPC mobilization.
Endothelium, uniquely positioned at the interface between the blood and the vessel wall, plays a crucial role in the physiology of circulation by performing multiple functions.1 It is involved in the regulation of coagulation, leukocyte adhesion in inflammation, transvascular flux of cells, liquids and solutes, vessel tone, and vascular smooth muscle growth. Endothelium also constitutes a target for the sex hormone 17β-estradiol (E2). In a series of experimental models, E2 has been reported to promote endothelial regrowth after endothelial denudation in rats2 and mice.3–5 These effects are known to be mediated through the estrogen receptor α3 and endothelial nitric-oxide synthase.4
Several growth factors could be involved in this process. Among them, in vitro models suggest that fibroblast growth factor-2 (FGF2) may be a key partner of E2.6,7 Moreover, both FGF2 and FGF receptor 1 are expressed in regenerating endothelial cells in a model of rat carotid injury,8 and FGF2 is an important mitogenic and angiogenic factor that stimulates endothelial cell growth, migration, and reendothelialization.9 FGF2 expression is complex because at least four isoforms (18, 22, 22.5, and 24 kd) in humans and three (18, 21, and 22 kd) in mice are synthesized, not through the classic mechanism of alternative splicing but through the alternative use of translation initiation codons.10–13
E2 was reported to stimulate p38 and p42/44 mitogen-activated protein kinase activity as well as cell migration and proliferation of cultured endothelial cells, all of these effects being dependent on estrogen receptor α but not on estrogen receptor β.14,15,16 Finally, we recently demonstrated that in cultured mouse endothelial cells, E2 increased both migration and proliferation in cells from wild-type Fgf2+/+ mice but not from mice disrupted for the Fgf2 gene (Fgf2−/−).7 Taken together, these data support an important role for FGF2 in the effect of E2 on migration and proliferation of endothelial cells, two key processes contributing to reendothelialization.
Reendothelialization was initially viewed as a local process consisting of migration and proliferation of endothelial cells from the adjacent uninjured area on each side.17,18 Since E2 is known to promote endothelial cell migration and proliferation in vitro,14–16 it was initially believed that E2 accelerated reendothelialization mainly through a local effect on adjacent uninjured endothelium. However, Asahara et al19 clearly demonstrated that vascular function not only depends on cells residing within the vessel wall but is also significantly modulated by circulating bone marrow (BM)-derived cells. A subset of these stem cells, endothelial progenitor cells (EPCs), enhances angiogenesis and vascular repair and diminishes atherosclerosis (Refs. 20–22). Furthermore, we5 and others4 have shown that E2 treatment enhances circulating EPC levels. In addition, both groups observed an increased EPC incorporation in carotid-injured areas.4,5 Finally, we also reported that intravenous injections of EPCs led to an increase in reendothelialization after endovascular injury.23 Altogether, these data support the idea that an increase in circulating EPC can contribute to acceleration of reendothelialization.20–22
The aim of the present study was to further explore the cellular and molecular mechanisms involved in the accelerative effect of E2 on reendothelialization. We used a model of electric perivascular injury, where reendothelialization occurs in an inflammatory context reminiscent of that of angioplasty of diseased arteries. We first found, using Fgf2−/− mice, that endogenous FGF2 is absolutely required for the effect of E2 both on the acceleration of reendothelialization and on the increase in circulating EPCs. As previously reported, we confirmed through an “en face” confocal microscopy approach the recruitment of BM-derived GFP (green fluorescent protein) cells into the regenerating endothelial monolayer. To evaluate the specific role of medullar and extramedullar FGF2 in these processes, we developed chimeric mice by grafting Fgf2+/+ BM to Fgf2−/− mice and vice versa. We found that the effect of E2 on reendothelialization and on EPC mobilization was lost when FGF2 expression in BM-derived cells is specifically abolished, whereas peripheral FGF2 alone was dispensable to mediate the E2 effect. Since medullar FGF2 seemed to play a crucial role in these processes, we studied the influence of E2 on the levels of FGF2 mRNA and protein isoforms in BM homogenates from Fgf2+/+.
Materials and Methods
Mice
Fgf2−/− mice were obtained as previously described.7,13 Fgf2+/+ and Fgf2−/− mice were initially maintained as an advanced intercross line on a mixed 129/SvEv × Black Swiss background. Fgf2−/− mice were then backcrossed sixfold with C57Bl/6J mice. This allowed study of the role of FGF2 deficiency in the effect of E2 on reendothelialization under this commonly used genetic background.
All mice were ovariectomized at 4 weeks of age and then implanted with pellets releasing either placebo or E2 (17β-estradiol, 0.1 mg for 60 days, ie, 80 μg/kg/day; Innovative Research of America, Sarasota, FL). The pellets were implanted s.c. in the back of the animals at 6 weeks of age (except in BM transplantation experiments, 10 weeks of age). All procedures were performed in accordance with the recommendations of the European Accreditation of Laboratory Animal Care Institute. Finally, C57BL/6-TgN(ACTbEGFP)1Osb transgenic littermates (GFP-Tg; Jackson Laboratories, Bar Harbor, ME) were also used as BM donor cells.24 In this transgenic line, with an enhanced GFP cDNA under the control of a chicken-actin promoter and cytomegalovirus enhancer (cac/enhanced GFP), all of the tissues, with the exception of erythrocytes and hair follicle cells, appear green under excitation light.24
We first confirmed the expression of the three isoforms of FGF2 in aorta, BM, and EPC extracts of Fgf2+/+ mice, and no band was detected in the corresponding extracts in Fgf2−/− mice, demonstrating the deficiency of FGF2 in these tissues and cell populations (Figure 1A). We then checked that placebo-treated ovariectomized mice had an atrophied uterus (<20 mg) and nondetectable (<5 pg/ml, ie, 20 × 10−12 M) circulating levels of E2, whereas those implanted with an E2-releasing pellet had a significant increase in uterine weight and serum E2 concentrations (100 to 200 pg/ml, not shown), irrespective of the genotype Fgf2+/+ and Fgf2−/− (Table 1). In addition, no variation of body weight was observed between Fgf2+/+ and Fgf2−/− mice (Table 1). Although E2 serum concentrations in this range have been reported by some authors during the estrous cycle,25,26 such concentrations are probably only reached during pregnancy.27
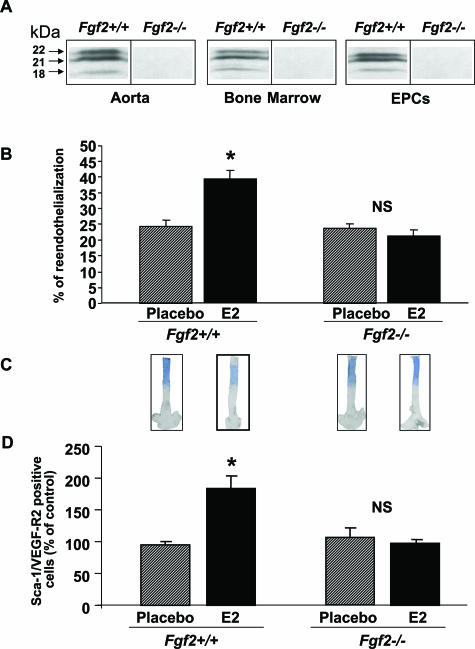
Figure 1.
Effect of estradiol (E2) on the reendothelialization process (3 days after injury) on EPC mobilization in ovariectomized Fgf2+/+ and Fgf2−/− mice treated (E2) or not (placebo) with E2. A: FGF-2 protein expression in aorta, bone marrow, and EPCs. B: The percentage of reendothelialization represents the ratio of the area remaining deendothelialized at day 3 on the area initially deendothelialized (eight mice in each group). *P < 0.05 versus respective control. C: Representative Evans blue dye staining of carotid 3 days after injury. D: EPC levels in peripheral blood were assessed from ovariectomized FGF2+/+ and FGF-2−/− mice treated (E2) or not (placebo) with E2 by FACS analysis to quantify Sca-1/VEGF-R2-positive cells (eight mice in each group, *P < 0.05 versus respective placebo).
Table 1.
Effect of E2 on the Body Weight, Uterine Weight, Reendothelialization Process (D3 and D5 after Injury), and Circulating EPC in Ovariectomized Fgf2+/+ or Fgf2−/− Mice
| Fgf2+/+ placebo n = 8 | Fgf2+/+ E2n = 8 | Fgf2−/− placebo n = 8 | Fgf2−/− E2n = 8 | Two-factor ANOVA FGF2
|
|||
|---|---|---|---|---|---|---|---|
| E2 | Genotype | Interaction | |||||
| Body weight (g) | 19.2 ± 0.4 | 17.6 ± 0.5 | 20.3 ± 0.3 | 20.9 ± 0.6 | NS | NS | NS |
| Uterine weight (mg) | <20 | 134.4 ± 10.2 | <20 | 140.6 ± 12.4 | |||
| % of reendothelialization at D3 | 23.4 ± 2.1 | 38.8 ± 1.6* | 22.8 ± 2.5 | 21.7 ± 2.0 | <0.001 | ||
| EPC in blood (% of control) | 95 ± 7 | 184 ± 22* | 106 ± 16 | 98 ± 7 | 0.002 | ||
| % of reendothelialization at D5 | 53.1 ± 3.2 | 79.1 ± 4.4* | 52.1 ± 4.5 | 49.1 ± 3.3 | <0.001 | ||
ANOVA, analysis of variance.
P < 0.05 versus respective control.
Irradiation and BM transplantation (Fgf2+/+BM = >Fgf2−/− and Fgf2−/−BM = >Fgf2+/+) did not alter uterus atrophy following ovariectomy nor did they increase uterine weight (Table 2) and serum E2 concentrations (not shown) in E2-treated mice. No variation of body weight was observed in these chimeric mice.
Table 2.
Effect of E2 on the Body Weight, Uterine Weight, Reendothelialization, and Circulating EPC in Ovariectomized Process in OvariectomizedFgf2−/− Grafted with Fgf2+/+ BM or Fgf2+/+ Grafted with Fgf2−/− BM Mice
| +/+ = >−/− Placebo n = 7 | +/+ = >−/− E2n = 7 | −/− = >+/+ Placebo n = 7 | −/− = >+/+ E2n = 7 | Two-factor ANOVA chimeric
|
|||
|---|---|---|---|---|---|---|---|
| E2 | Genotype | Interaction | |||||
| Body weight (g) | 22.6 ± 0.3 | 22.1 ± 0.8 | 20.1 ± 0.4 | 22.1 ± 0.6 | NS | NS | NS |
| Uterine weight (mg) | <20 | 136.6 ± 10.0 | <20 | 128.6 ± 7.8 | |||
| % of reendothelialization at D3 | 29.7 ± 1.5 | 47.9 ± 1.6* | 27.1 ± 1.6 | 30.8 ± 2.3 | <0.0001 | ||
| EPC in blood (% of control) | 24 ± 3 | 149 ± 64* | 27 ± 3 | 33 ± 12 | 0.04 | ||
The percentage of reendothelialization represents the ratio of the area remaining deendothelialized at D3 or D5 on the area initially deendothelialized.
ANOVA, analysis of variance.
P < 0.05 versus respective control.
Bone Marrow Transplantation
Two weeks after ovariectomy, recipient mice were lethally irradiated (9 Gy, g-source), after which they received an intravenous injection of 4 × 106 donor BM cells 24 hours later. One month later, mice were implanted with either a placebo or an E2 pellet.
Electric Injury
Three weeks after the pellet implantation, mice were submitted to an electric injury at the level of the left common carotid artery as previously described28.3 This technique allows precise deendothelialization of 4-mm length and measurement of the remaining deendothelialized area at day 3 (and at day 5 for Fgf2+/+ and Fgf2−/− mice). To measure the percentage of reendothelialization, animals were perfused in vivo with Evans blue dye to identify the remaining denuded area (Figure 1C). Then, a ratio was calculated from the remaining denuded area to deendothelialized area at day 0. In additional experiments, silver nitrate staining of en face preparation was also performed at day 10 to verify that reendothelialization was complete at that time (not shown).
Serum Hormone Concentrations
Radioimmunoassay kits for E2 were used following the manufacturer’s instructions (Sorin Biomedica, Saluggia, Italy).
Fluorescence-Activated Cell Sorter Analysis
Peripheral blood and BM from mice were collected after 3 weeks of placebo or E2 treatment in vivo. After lysis of blood red cells and Fc blockade (Fc-Block; Pharmingen, San Diego, CA), the viable lymphocyte population was analyzed for the expression of Sca-1-fluorescein isothiocyanate (clone E13–161.7; Pharmingen) and vascular endothelial growth factor receptor-2 (clone A3; Santa Cruz Biotechnology, Santa Cruz, CA) conjugated with the corresponding phycoerythrin-labeled secondary antibody (Sigma, St. Louis, MO). Isotype-identical antibodies served as controls (Becton, Dickinson and Company, San Jose, CA). Single- and two-color flow cytometric analyses were performed by using a Becton Dickinson FACScan equipped with an argon ion laser. Data were evaluated with Cellquest software.
Western Blotting and Quantification of FGF2 mRNA
Electrophoresis was performed as described by Laemmli on 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels under reducing conditions. Cellular extracts (50 μg) were loaded on gels. Mouse monoclonal anti-Fgf2 (sc-79; Santa Cruz Biotechnology) and monoclonal anti-β-actin (AC-15; Sigma) were used at the concentration of 1 μg/ml. HRP-labeled secondary antibodies were revealed by the ECL system (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK).
Extraction of Bone Marrow mRNA, Reverse-Transcription-Polymerase Chain Reaction (RT-PCR), and Quantitative PCR
Extraction of bone marrow mRNA was performed with the RNAble system (Eurobio, Courtaboeuf, France), according to the manufacturer’s instructions. Samples were treated for DNA contamination by DNA digestion during RNA purification (Sigma). One μg of total mRNA was use for RT, performed with random hexamers and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA), and subjected to real-time quantitative PCR using a Taqman 7900 Detection System quantitative PCR (SYBR Green Jumpstart Taq ready mix; Sigma). All primers were designed using the PRIMER express program (FGF2: 5′-CACCAGGCCACTTCAAGGA-3′ and 3′-GATGGATGCGCAGGAAGAA-5′). The expression of FGF2 mRNA was normalized to RNA loading for each sample using 18S rRNA as an internal standard (18S: 5′-CAACTAAGAACGGCCATGCA-3′ and 3′-AGCCTGCGGCTTAATTTGAC-5′).
En Face Confocal Microscopy
Intravascular blood was first removed with an intracardiac injection of phosphate-buffered saline. After sampling, carotids were carefully taken and fixed for 20 minutes in phosphate-buffered saline containing 4% paraformaldehyde. Carotids were then longitudinally opened, and fixation was quenched with 100 mmol/L glycine (pH 7.4), and samples were permeabilized for 10 minutes in 0.1% Triton X-100 and washed in phosphate-buffered saline. Fixed tissues were blocked with solution I (75 mM NaCl, 18 mM Na3 citrate, 2% goat serum, 1% bovine serum albumin, 0.05% Triton X-100, and 0.02% NaN3) for 2 hours at room temperature. Von Willebrand antibody (Dako, Trappes, France) was diluted in solution I (1:500) and incubated with carotids for 48 hours at 4°C. Then, tissues were washed with solution II (75 mM NaCl, 18 mM sodium citrate, and 0.05% Triton X-100) for 2 hours and rinsed in phosphate-buffered saline. Carotids were then incubated for 1 hour at room temperature with fluorophore-conjugated antibodies (goat anti-rabbit Alexa 633, 5 μg/ml; Molecular Probes, Eugene, OR) diluted in solution I. Finally, tissues were washed in solution I for 1 hour at room temperature. To label the nuclei, propidium iodide was used (2 μg/ml; Sigma). All preparations were mounted with Kaiser’s glycerol gelatin (Merck, Darmstadt, Germany). All microscopy imagery was performed with ZEISS LSM 510 (Carl Zeiss GmbH, Jena, Germany).
Statistics
Results are expressed as means ± SEM. To test the respective roles of E2 treatment, Fgf2 genotype, and chimerism on reendothelialization or on circulating EPCs, a two-factor analysis of variance was performed in the two sets of experiments. When an interaction was observed between the two factors, the four groups were compared using a one-factor analysis of variance. The results of this test are indicated by an asterisk when P < 0.05 to facilitate the reading of the figures. P < 0.05 was considered statistically significant.
Results
FGF2 Is Absolutely Required for the Effect of E2 on Reendothelialization and EPC Mobilization
Reendothelialization was evaluated in vivo after electric injury of carotid artery. As shown in Table 1 and Figure 1B, basal endothelial regeneration was similar in ovariectomized Fgf2+/+ and Fgf2−/− mice implanted with a placebo pellet. To test the respective roles of E2 treatment and FGF2 genotype on reendothelialization at day 3, we performed a two-factor analysis of variance, which revealed a significant interaction (P < 0.001). Indeed, E2 treatment significantly accelerated reendothelialization in Fgf2+/+ mice (P < 0.005) but had no effect in Fgf2−/− mice (P = 0.41, NS) (Figure 1B). Similar results were obtained at day 5 (Table 1), demonstrating that FGF2 is absolutely necessary for the E2-mediated acceleration of reendothelialization. As the level of significance of the accelerative effect of E2 on reendothelialization was similar at day (D) 3 and D5, we decided to study only D3 in the rest of the study.
To test whether FGF2 was also involved in EPC mobilization in response to E2, EPC was measured in blood by FACScan analysis using two specific markers, Sca-1 and vascular endothelial growth factor receptor-2, as previously described.5 A two-factor analysis of variance revealed a significant interaction between E2 treatment and FGF2 genotype (P < 0.001). As shown in Figure 1D and Table 1, E2 increased circulating EPC levels in peripheral blood in Fgf2+/+ mice, whereas it did not alter them in Fgf2−/− mice, demonstrating that FGF2 is also absolutely required for EPC mobilization in response to E2.
Bone Marrow FGF2 Is Required for the Effect of E2 on Reendothelialization and on EPC Mobilization
To test whether the effect of E2 on reendothelialization and on EPC mobilization was dependent on FGF2 expression in BM cells or in extramedullar cell populations, we generated chimeric mice by grafting Fgf2+/+BM to Fgf2−/− mice and vice versa. It is of importance to note that radiation and bone marrow grafting affected neither the basal nor the E2 effect on the reendothelialization process in this electrical injury model (not shown).
As shown in Table 2 and Figure 2A, endothelial regeneration was similar in ovariectomized mice Fgf2+/+BM = >Fgf2−/− and Fgf2−/−BM = >Fgf2+/+ mice implanted with a placebo pellet. The two-factor analysis of variance revealed an interaction (P < 0.001) between E2 treatment and the type of FGF2 deficiency on reendothelialization. Indeed, E2 treatment significantly accelerated reendothelialization in Fgf2+/+BM = >Fgf2−/− mice (P < 0.001) but had no effect in Fgf2−/−BM = >Fgf2+/+ mice (P = 0.34, NS).
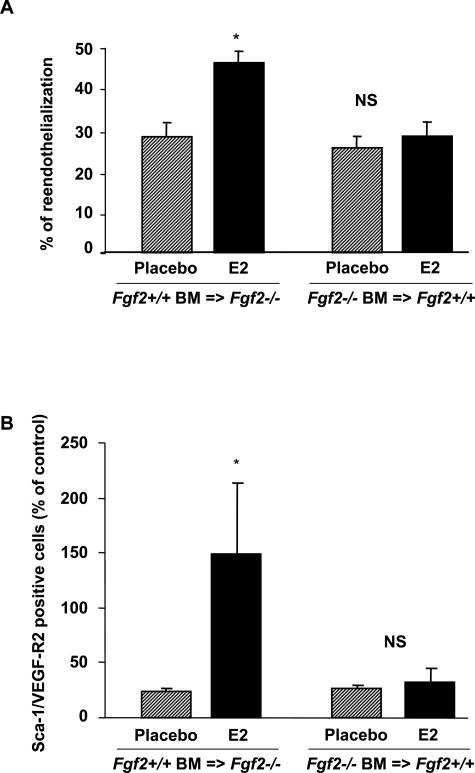
Figure 2.
Effect of E2 on reendothelialization (A) and on the EPC mobilization in chimeric mice (B) in ovariectomized Fgf2−/− grafted with Fgf2+/+BM (Fgf2+/+BM = >Fgf2−/−) and Fgf2+/+ grafted with Fgf2−/−BM (Fgf2−/−BM = >Fgf2+/+) mice treated (E2) or not (placebo) with E2. The percentage of reendothelialization represents the ratio of the area remaining deendothelialized at day 3 on the area initially deendothelialized. EPC levels in peripheral blood EPCs assessed by FACS analysis to quantify Sca-1/VEGF-R2-positive cells (seven mice in each group). *P < 0.05 versus negative control.
The number of EPCs was analyzed in blood of chimeric mice to determine the involvement of BM FGF2 with respect to that of other cell origins in EPC mobilization in response to E2. As shown in Figure 2B and Table 2, E2 increased EPC levels in peripheral blood in chimeric Fgf2+/+BM = >Fgf2−/− mice. In contrast, in chimeric Fgf2−/−BM = >Fgf2+/+ mice, this effect was absent. Altogether, these results show that FGF2 expression in BM is required for the effect of E2 on both reendothelialization and EPC mobilization.
Effect of E2 on FGF2 Expression
As previously reported,13 FGF2 isoforms are expressed in various tissues including BM, aorta (Figure 3), and lung (not shown). E2 treatment markedly induced both FGF2lmw (2.5-fold, 38 ± 4 versus 95 ± 5 densitometric units and FGF2hmw (9-fold, 10 ± 4 versus 90 ± 3 densitometric units) protein levels in BM homogenates from Fgf2+/+ mice (Figure 3A). Unexpectedly, the abundance of FGF2 mRNA was decreased sevenfold in the BM (Figure 3A).
Figure 3.
FGF2 isoform abundance and FGF2 mRNA expression in bone marrow (A) and in aorta (B) from Fgf2+/+ mice A: Representative Western blot analysis showed that E2 increased both FGFlmw and FGFhmw (quantification was the sum of 21- and 22-kd bands, performed on four separate Western blots) isoforms in BM homogenates. In contrast, abundance of FGF2 mRNA in BM was decreased by E2. *P < 0.05 versus respective placebo. B: Quantification of Western blot showed that only FGF2hmw isoform are increased in aorta homogenates. *P < 0.05 versus respective placebo.
In addition, FGF2hmw isoforms were more abundant than the FGF2lmw protein both in aorta (Figure 3B) and in lung (not shown) homogenates from untreated Fgf2+/+ mice. E2 treatment preferentially increased FGF2hmw protein levels both in aorta (1.4-fold, Figure 2B) and in lung (1.5-fold, not shown) homogenates from Fgf2+/+ mice. FGF2 protein abundance in injured carotid artery homogenates was below the threshold level using Western blot analysis (not shown).
To further characterize the relationship between BM-derived cells and the regenerating endothelial cell monolayer, longitudinally opened and flattened mouse carotid artery was observed using en face confocal microscopy. To visualize cells originating from BM at the level of the injured carotid, we grafted GFP-Tg BM to wild-type mice. At day 3 (Figure 4A), GFP-expressing cells were detected at the level of the regenerated endothelial cell monolayer as well as in abundance in the adventitia of the injured vessel (Figure 4B), confirming the homing of FGF2+/+ BM-derived cells in injured carotid arteries. However, FGF2 protein could not be detected using confocal microscopy or classical immunohistochemistry (not shown).
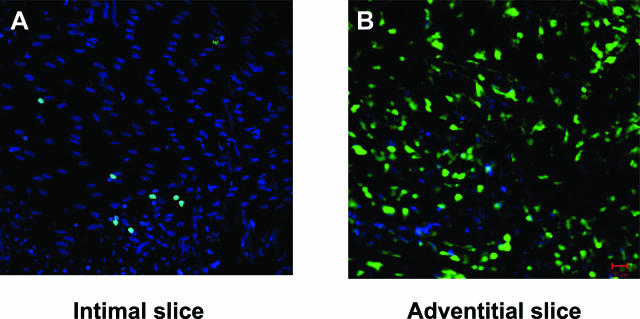
Figure 4.
Localization of BM-derived cells by “en face” confocal microscopy in the injured carotid artery of chimeric mice. At day 3 after injury, BM-derived GFP-positive cells were detected at the level of the intima (A), as well as abundantly in the adventitia (B).
Discussion
The main findings of the present work are threefold. 1) E2 accelerates reendothelialization and increases EPC mobilization through an FGF2-dependent mechanism. This is the first in vivo evidence of the implication of FGF2 as a mediator an in vivo effect of E2. 2) Only medullar, but not extramedullar, FGF2 is required for both effects of E2, demonstrating for the first time that an accelerative effect of reendothelialization requires the involvement of bone marrow. 3) E2 impressively increased FGF2 isoforms in bone marrow.
Endovascular deendothelialization in mice is technically difficult because of the very small size of the carotid artery. Carmeliet et al28 proposed a method in which the endothelium is destroyed through a perivascular electric injury, and an adaptation of this model allowed us to demonstrate previously that E2 accelerates reendothelialization through estrogen receptor α.3 Endovascular and electric perivascular injuries are identical in their efficiency to destroy the endothelium, but they differ significantly in their effects on the underlying cell population in the vessel wall. Endovascular injury preserves most of the adventitial cells as well as most of the medial smooth muscle cells, which remain a potential cellular partner.29
Using the model of endovascular carotid injury, we previously showed that the constitution of neointima hyperplasia was not influenced by FGF2 deficiency, supporting the idea that endogenous FGF2 is not involved in spontaneous arterial healing.13 This parameter could not be assessed in the present study because the carotid electric injury model does not elicit neointima hyperplasia,3,28 probably due to the destruction of medial smooth muscle cells. It is now clear that the effects of E2 are not limited to the field of reproductive physiology but have many other effects, in particular in the pathophysiology of arteries.30,31 In striking contrast to the absence of consequence of FGF2 deficiency on basal reendothelialization (Figure 1), we show here, for the first time to our knowledge, that endogenous FGF2 is an absolute requirement for the acceleration of this process in response to E2.
As nicely demonstrated by Schwartz, Haudenschild, and others 20 years ago,17,18 the acceleration of reendothelialization by E2 could be the consequence of an increase in migration and proliferation of endothelial cells from each side of the adjacent uninjured area. Since E2 increases endothelial cell migration in vitro,14–16 acceleration of reendothelialization by E2 could be the consequence of the direct effect of the hormone on the in situ regenerating endothelium. This possibility was further supported by the fact that endogenous FGF2 is required for the stimulatory effect of E2 on migration and proliferation of cultured endothelial cells7 as well as for the acceleration of reendothelialization by E2 in vivo (Figure 1). The modest but significant induction of both FGF2hmw in the intact aorta in response to E2 (present work), in line to the induction of FGF2hmw in cultured endothelial cells by E2,7 was consistent with the idea of a local regulation of the reendothelialization process by E2.
However, Asahara et al19 clearly demonstrated that vascular function not only depends on cells residing within the vessel wall but is also significantly modulated by circulating BM-derived cells, opening a new area of research. E2 was shown to increase the number of circulating EPCs. As previously shown,4,5 we confirm that E2 increases EPC mobilization in Fgf2+/+ mice and demonstrate the abolition of this effect in Fgf2−/− mice (Figure 1). Of major interest, we developed chimeric mice by grafting Fgf2−/−BM to Fgf2+/+ (Fgf2−/−BM = >Fgf2+/+) mice and vice versa and demonstrated that bone marrow FGF2 is absolutely required for EPC mobilization by E2, whereas extramedullary FGF2 is dispensable for this effect (Figure 2).
The same chimeric models indicated that bone marrow FGF2, but not extramedullary FGF2, is also necessary for the acceleration of reendothelialization by E2, supporting the hypothesis that EPC mobilization could be determinant in the healing effect of E2 (Figure 2B). In addition, it appeared that extramedullary FGF2 is dispensable for the accelerative effect of E2 on reendothelialization, excluding a role for the FGF2 expressed in the adjacent uninjured endothelium in this process.
In the present work, E2 treatment markedly increased FGF2 isoforms levels in BM homogenates from Fgf2+/+ mice (Figure 3A). At the same time, E2 treatment was found to markedly decrease FGF2 mRNA abundance in the same tissues (Figure 3A). Such a discrepancy has been previously reported by us in 3T3 cells32 and by others in erythroid cells.33 This strongly suggests a regulation at the translational level. Indeed, when the ribosomal machinery mobilizes the pool of silent mRNA, its translation could activate its instability, possibly by removing masking-protein able to protect the instability region.33 Such a region has been clearly identified within the 3′-end of Fgf2 mRNA.34 Furthermore, we have previously identified a translational enhancer located at the end of the 3′-untranslated region of the Fgf2 gene, the activation of which promotes a preferential increase of the FGF2hmw forms.35 These two phenomena involving the role of the 3′-untranslated region of Fgf2 mRNA could explain the effect observed here after stimulation by E2. Interestingly, the systematic analysis of gene expression in yeast clearly showed the lack of correlation between mRNA and protein levels, showing that this discrepancy between mRNA and protein abundance could be the rule rather than the exception in genes expressed at a low level.36,37
We previously demonstrated that estrogens increase the number of EPCs by exerting antiapoptotic effects, and suggested that this could lead to accelerated vascular repair and decreased neointima formation in the model of endovascular carotid artery injury.5 Furthermore, we5 and others4 have shown an increased incorporation of BM-derived cells in the reendothelialized area. In the present study, the effects of E2 on circulating EPCs and on the acceleration of reendothelialization correlated in Fgf2+/+ and Fgf2−/− mice as well as in chimeric Fgf2+/+/Fgf2−/− mice (Figures 1 and 2), further suggesting that EPCs participate in accelerating reendothelialization. The development of “en face” confocal microscopy allowed us to confirm the presence of GFP-expressing cells (potentially EPCs) present at the level of the regenerated endothelial monolayer (Figure 4A) but also of the adventitia (Figure 4B). The respective role of both of these BM-derived populations on the reendothelialization process remains to be determined.
Although the phenotype of Fgf2−/− mice suggests a nonessential role of FGF2 in basal reendothelialization and in angiogenesis during reproduction and development, the present results demonstrate for the first time an essential role of FGF2 in the effect of E2 on reendothelialization and EPC mobilization. The induction of FGF2 expression by E2 in bone marrow is, to our knowledge, the strongest physiological regulation reported so far. More specifically, BM FGF2 appears to be a key determinant of the effect of E2 on the reendothelialization process, as well as on EPC mobilization. These functions represent novel insight into the physiological roles attributable to FGF2. In addition, the role of FGF2 may not be restricted to the effect of E2, and other major vasculoprotective interventions such as exercise or statins, both of which are known to share several mechanisms with E2 and to mobilize EPCs,4,20,38,39 could also rely on FGF2 to mediate their effect. Furthermore, FGF2 could also be involved in the beneficial effects of exercise or statins on reendothelialization and arterial repair. These hypotheses should be tested in future studies. A better understanding of the role of FGF2 in pathophysiology could help to design or optimize novel strategies for therapeutic intervention.
Acknowledgments
We thank Dr. Françoise Bono (Sanofi Laboratories) for the gift of the C57Bl/6 background FGF2-deficient mice.
Footnotes
Address reprint requests to JF Arnal, INSERM U589, Institut L. Bugnard, CHU Rangueil, TSA 50032, 31059 Toulouse Cedex, France. E-mail: arnal@toulouse.inserm.fr.
The work was supported in part by INSERM, Université Paul Sabatier Toulouse III, the European Vascular Genomics Network No. 503254, the Agence Nationale de la Recherche, the Fondation de France, the Fondation de l’Avenir, and the Conseil Régional Midi-Pyrénées in France. V.F. was supported by a grant from the Nouvelle Société Française d’Athérosclérose.
V.F. and C.F. contributed equally to this work.
References
- Alexander RW, Dzau VJ. Vascular biology: the past 50 years. Circulation. 2000;102:IV112–IV116. doi: 10.1161/01.cir.102.suppl_4.iv-112. [DOI] [PubMed] [Google Scholar]
- Krasinski K, Spyridopoulos I, Asahara T, van der Zee R, Isner JM, Losordo DW. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation. 1997;95:1768–1772. doi: 10.1161/01.cir.95.7.1768. [DOI] [PubMed] [Google Scholar]
- Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Bohm M, Nickenig G. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- Kim-Schulze S, Lowe WL, Jr, Schnaper HW. Estrogen stimulates delayed mitogen-activated protein kinase activity in human endothelial cells via an autocrine loop that involves basic fibroblast growth factor. Circulation. 1998;98:413–421. doi: 10.1161/01.cir.98.5.413. [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Delmas E, Gourdy P, Zhou M, Bossard C, Bugler B, Bayard F, Krust A, Prats AC, Doetschman T, Prats H, Arnal JF. Role of fibroblast growth factor-2 isoforms in the effect of estradiol on endothelial cell migration and proliferation. Circ Res. 2004;94:1301–1309. doi: 10.1161/01.RES.0000127719.13255.81. [DOI] [PubMed] [Google Scholar]
- Lindner V, Reidy MA. Expression of basic fibroblast growth factor and its receptor by smooth muscle cells and endothelium in injured rat arteries. An en face study. Circ Res. 1993;73:589–595. doi: 10.1161/01.res.73.3.589. [DOI] [PubMed] [Google Scholar]
- Lindner V, Majack RA, Reidy MA. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990;85:2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkiewicz RZ, Sommer A. Human basic fibroblast growth factor encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci USA. 1989;86:3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats H, Kaghad M, Prats AC, Klagsbrun M, Lelias JM, Liauzun P, Chalon P, Tauber JP, Amalric F, Smith JA, Caput D. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci USA. 1989;86:1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud E, Touriol C, Boutonnet C, Gensac MC, Vagner S, Prats H, Prats AC. A new 34-kilodalton isoform of human fibroblast growth factor 2 is cap dependently synthesized by using a non-AUG start codon and behaves as a survival factor. Mol Cell Biol. 1999;19:505–514. doi: 10.1128/mcb.19.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, Yin M, Coffin JD, Kong L, Kranias EG, Luo W, Boivin GP, Duffy JJ, Pawlowski SA, Doetschman T. Fibroblast growth factor 2 control of vascular tone. Nat Med. 1998;4:201–207. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DE, McGowan KA, Grant DS, Maheshwari S, Bhartiya D, Cid MC, Kleinman HK, Schnaper HW. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995;91:755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Sirois MG, Tanguay JF. Specific contribution of estrogen receptors on mitogen-activated protein kinase pathways and vascular cell activation. Circ Res. 2003;93:399–405. doi: 10.1161/01.RES.0000088640.18462.42. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Sirois MG, Bernatchez PN, Tanguay JF. Estrogen regulation of endothelial and smooth muscle cell migration and proliferation: role of p38 and p42/44 mitogen-activated protein kinase. Arterioscler Thromb Vasc Biol. 2002;22:1585–1590. doi: 10.1161/01.atv.0000035393.11854.6a. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Haudenschild CC, Eddy EM. Endothelial regeneration. I. Quantitative analysis of initial stages of endothelial regeneration in rat aortic intima. Lab Invest. 1978;38:568–580. [PubMed] [Google Scholar]
- Haudenschild CC, Schwartz SM. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979;41:407–418. [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol. 2004;287:C572–C579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Witchell CM, Metcalfe JC. Tamoxifen elevates transforming growth factor-beta and suppresses diet-induced formation of lipid lesions in mouse aorta. Nat Med. 1995;1:1067–1073. doi: 10.1038/nm1095-1067. [DOI] [PubMed] [Google Scholar]
- Sullivan TR, Jr, Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ, O’Donnell TF, Jr, Mendelsohn ME. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995;96:2482–2488. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman MD, Schachter BS, Karelus K, Combatsiaris EP, Garcia T, Nelson JF. Up-regulation of the uterine estrogen receptor and its messenger ribonucleic acid during the mouse estrous cycle: the role of estradiol. Endocrinology. 1992;130:1923–1930. doi: 10.1210/endo.130.4.1547720. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Stassen J, De Mol M, Bouché A, van den Oord J, Kocks M, Collen D. Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol. 1997;150:761–776. [PMC free article] [PubMed] [Google Scholar]
- Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6–12. doi: 10.1161/01.atv.21.1.6. [DOI] [PubMed] [Google Scholar]
- Hodgin J, Maeda N. Estrogen and mouse models of atherosclerosis. Endocrinology. 2002;143:4495–4501. doi: 10.1210/en.2002-220844. [DOI] [PubMed] [Google Scholar]
- Galy B, Creancier L, Zanibellato C, Prats AC, Prats H. Tumour suppressor p53 inhibits human fibroblast growth factor 2 expression by a post-transcriptional mechanism. Oncogene. 2001;20:1669–1677. doi: 10.1038/sj.onc.1204271. [DOI] [PubMed] [Google Scholar]
- Weiss IM, Liebhaber SA. Erythroid cell-specific mRNA stability elements in the alpha 2-globin 3′ nontranslated region. Mol Cell Biol. 1995;15:2457–2465. doi: 10.1128/mcb.15.5.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touriol C, Morillon A, Gensac MC, Prats H, Prats AC. Expression of human fibroblast growth factor 2 mRNA is post-transcriptionally controlled by a unique destabilizing element present in the 3′-untranslated region between alternative polyadenylation sites. J Biol Chem. 1999;274:21402–21408. doi: 10.1074/jbc.274.30.21402. [DOI] [PubMed] [Google Scholar]
- Touriol C, Roussigne M, Gensac MC, Prats H, Prats AC. Alternative translation initiation of human fibroblast growth factor 2 mRNA controlled by its 3′-untranslated region involves a Poly(A) switch and a translational enhancer. J Biol Chem. 2000;275:19361–19367. doi: 10.1074/jbc.M908431199. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes G, Carter MS, Eisen MB, Brown PO, Sarnow P. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc Natl Acad Sci USA. 1999;96:13118–13123. doi: 10.1073/pnas.96.23.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]