Abstract and Introduction
Abstract
Irritable bowel syndrome (IBS) is a highly prevalent gastrointestinal motility disorder broadly characterized by abdominal pain/discomfort associated with altered bowel habits. The chronic and bothersome nature of IBS symptoms often negatively affects patient quality of life and activity level and places a substantial economic burden on patients and the healthcare system. Advances in research have led to a greater understanding of the underlying pathophysiology of IBS, particularly regarding the role serotonin plays in the gastrointestinal tract; the development of stepwise, symptom-based diagnostic strategies that allow for a diagnosis of IBS to be made without the need for extensive laboratory testing; and the development of treatment options targeting underlying pathophysiologic mechanisms that provide relief of the multiple symptoms associated with IBS. This review highlights recent advances in research and discusses how these findings can be applied to daily clinical practice.
Introduction
IBS – a complex, multifaceted condition broadly characterized by abdominal pain/discomfort associated with altered bowel habits – is among the most prevalent gastrointestinal (GI) motility disorders. Prevalence estimates for IBS range from 3% to 20%, with most estimates in North America ranging from 10% to 15%.[1–3] Women are affected by IBS more often than men (2:1 in the community setting and 3:1 to 4:1 in the tertiary care setting).[2] IBS-related symptoms are often chronic and bothersome, negatively affecting patient activities of daily living (eg, sleep, leisure time), social relationships, and productivity at work or school.[4–6] Patients with IBS typically score lower than population norms or those with other chronic GI and non-GI disorders on measures of quality of life.[7–10] IBS also puts a heavy economic burden on patients, employers, and the healthcare system, resulting in more than $10 billion in direct costs (eg, from office visits, medications) and $20 billion in indirect costs (eg, through work absenteeism and reduced productivity) each year.[11–14]
Advances in research during the past several decades have provided insight into the underlying pathophysiology of IBS, particularly the role of serotonin in the GI tract; the development of stepwise, symptom-based diagnostic strategies; and the development of targeted treatment options. This review discusses recent advances in research and explores how these findings can be applied in the clinical practice setting.
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eye only or for possible publication via email: glundberg@medscape.net
Making a Positive Diagnosis of IBS
IBS is not associated with any definitive biochemical, structural, or serologic abnormalities that define its presence. The hallmark feature of IBS is abdominal pain or discomfort associated with altered bowel habits, and, often, the abdominal pain prompts patients to seek medical care. Because the symptoms of IBS are common to a number of other GI conditions, IBS was long considered a “diagnosis of exclusion,” leading to excessive testing of patients with characteristic symptoms. Fortunately, advances in research have led to the development of symptom-based approaches, aimed at standardizing IBS patient subgroups, and the development of consensus guidelines advocating a positive diagnosis of IBS based primarily on the pattern and nature of symptoms, without the need for excessive laboratory testing.[2,15]
In 1978, Manning and colleagues[16–18] proposed diagnostic criteria for IBS that were found to be reasonably sensitive and specific. More recently, an international group of experts in functional GI motility disorders convened to develop symptom-based criteria, known as the Rome criteria (Rome I,[19] Rome II,[15] and Rome III[20,21]), to better define and provide tools by which a positive diagnosis of these disorders could be made. The Rome I criteria for IBS were shown to be sensitive and specific.[22,23] The Rome II criteria, published in 1999,[15] were intended to simplify the more complex Rome I criteria by better defining the nonconsecutive, multisymptom nature of this disorder. Clinical studies validating the Rome II criteria are beginning to emerge.[24] Consensus has not yet been reached regarding which of the Rome criteria are more sensitive for identifying IBS patients in clinical practice[22,24,25] and which of the 2 more accurately estimates the prevalence of IBS.[17,26,27] A recent US population-based follow-up study found that the Rome II criteria were limited in capturing the fluctuating nature of IBS. Over a 2-year period, patients experienced symptoms episodically, including abdominal pain; the absence of pain at time of follow-up excluded many patients from meeting IBS criteria.[28] Overall, the Rome I and II criteria are considered useful for standardizing enrollment of patients into clinical trials. However, many clinicians believe that these criteria are too restrictive for use in clinical practice. The American College of Gastroenterology (ACG) Functional GI Disorders Task Force suggests using a broader definition of IBS: abdominal discomfort associated with altered bowel habits.[2] The Rome III working team met in the fall of 2004; updated criteria were published in April 2006. The principle difference between Rome III guidelines as compared with the Rome II criteria lies in the less restrictive timeframe for symptoms. Whereas the Rome II criteria require symptoms to be present for at least 12 weeks (not necessarily consecutive) in the past 12 months, the Rome III criteria require symptoms to originate for 6 months prior to diagnosis, and be currently active (ie, patient meets criteria) for 3 months. The categorization of IBS patients into the constipation, diarrhea, or mixed subtypes has also been revised (based on stool consistency).[20] It is hoped that the less restrictive symptom timeframe requirements of the Rome III guidelines will make them more clinically practical than the previous iterations.
The Rome III criteria, in conjunction with careful history-taking (medical, family, and medication) and thorough physical examination, can be applied as part of the stepwise, symptom-based approach to diagnosing IBS (Figure). The presence of alarm features (eg, rectal bleeding, history of colon cancer or inflammatory bowel disease) potentially indicative of organic disease necessitates further evaluation.[2]
Figure.
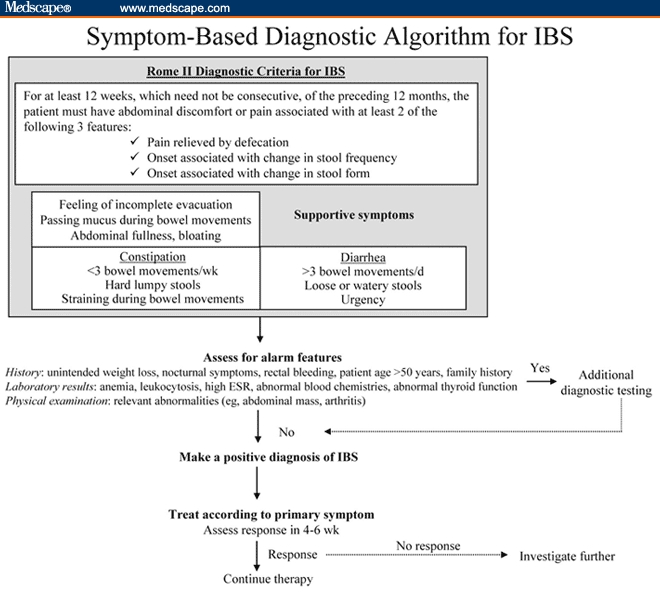
Until recently, the role of diagnostic testing in diagnosing IBS was a topic of continuing debate. Recent evidence indicates that when a patient meets the Rome criteria, and has no features suggestive of organic disease, the pretest probability of making an accurate diagnosis of IBS based primarily on symptoms is high. In a systematic review conducted by Cash and colleagues,[32] the pretest probability of inflammatory bowel disease, colorectal cancer, or infectious diarrhea was found to be less than 1% in patients meeting symptom-based criteria for IBS. Extensive diagnostic testing rarely identifies organic GI disease in these patients. However, one exception is celiac disease: the pretest probability of celiac disease in patients meeting symptom-based criteria for IBS was 10 times higher than that in the general population.[32] Recently, testing for celiac disease in patients with suspected IBS with diarrhea (IBS-D) has been shown to be most financially feasible in areas in which the prevalence of celiac disease is at least 8%.[33] These findings were supported by the evidence-based recommendation made by the ACG Functional GI Disorders Task Force, whereby they concluded that there is insufficient evidence to recommend the routine performance of diagnostic testing in patients who meet symptom-based criteria for IBS (eg, Manning, Rome I, Rome II) – therefore, supporting a symptom-based approach to making a positive diagnosis of IBS.[2]
The extent to which this guidance is implemented into clinical practice is based on clinical judgment and experience of the physicians. Most physicians agree that standard laboratory testing, such as complete cell blood count, chemistry panel, and erythrocyte sedimentation rate, should be performed in patients with symptoms of IBS. However, if these studies have been performed recently, after symptoms emerged, there is no need to repeat them. For a patient with IBS symptoms and no comorbid conditions, test results should all be within normal limits. Colonoscopy is generally not required in the absence of features indicative of organic disease (eg, family history of colon cancer), although it should be performed in individuals older than 50 years of age.[1] However, as mentioned previously, if the patient (regardless of age) underwent colonoscopy after the development of symptoms, a repeat test is not warranted. In addition to routine laboratory testing, thyroid function studies, ova and parasite examination, urinalysis, and breath tests for lactose intolerance may be considered. Globally, however, the value of diagnostic testing in IBS has not been established in patients who meet symptom-based criteria for IBS and who do not have symptoms indicative of organic disease; additional diagnostic testing does not increase the probability of detecting organic disease and does not alter the diagnosis of IBS once it is made.[32,34–36]
A symptom-based diagnosis of IBS is durable. In a longitudinal study in which 75 patients were reevaluated 10 to 13 years after an initial IBS diagnosis, all patients still met the diagnostic criteria for IBS (Manning, Rome I, Rome II) and few had received an alternative diagnosis during that period.[37] In other studies with follow-up periods ranging from 3 to 5 years, development of organic disease occurred in less than 7% of patients initially given a diagnosis of IBS using the symptom-based approach.[38]
Historically IBS was considered a diagnosis by exclusion and was viewed as a purely psychosomatic condition. Within the past 2 decades, however, symptom-based diagnostic criteria have been established that allow physicians to positively and confidently diagnose IBS.
The Science of IBS
Given the lack of definitive organic markers for IBS, the absence of a unifying hypothesis regarding its underlying pathophysiology is not surprising. Nevertheless, important advances in research made during the past 50 years have brought us closer than ever to understanding the numerous putative etiologic factors involved in this multifaceted disorder, including environmental factors, genetic links, previous infection, food intolerance, and abnormal serotonergic signaling in the GI tract.
Environmental Influences
Although a patient's psychological state may influence the way in which he or she presents, copes with illness, and responds to treatment, no evidence supports the theory that psychological disturbances are the cause of IBS.[39,40] The biopsychosocial model proposed by Engel takes into account the interplay between biologic, psychological, and social factors.[41] This model proposes that there is an underlying biologic predisposition for IBS that may be acted on by environmental factors and psychological stressors, which contribute to disease development, the patient's perception of illness, and impact on treatment outcomes.[42,43]
Studies evaluating the role of acute stress have shown that stress can result in release of stress-related hormones that affect colonic sensorimotor function (eg, corticotropin-releasing factor [CRF] and inflammatory mediators [eg, interleukin (IL)-1]), leading to inflammation and altering GI motility and sensation.[44] For example, CRF-1 receptors located in the central nervous system (CNS) and gut can affect colonic motility, epithelial water transport, and gut permeability.[45] Sagami and colleagues[46] determined that the peripheral administration of a nonselective corticotropin-releasing hormone (CRH) receptor antagonist improved GI motility, visceral perception, and negative mood in response to gut stimulation in patients with IBS. These findings suggest that CRH may play an important role in the pathophysiology of IBS.
Genetics
Studies with twins have shown that IBS is twice as prevalent in monozygotic twins as in dizygotic twins.[47–49] Limited research on familial aggregation has found that individuals who have a family member (other than a spouse) with a history of abdominal pain or bowel disorder have more than 2-fold increased odds of having IBS. It is likely that environmental influences may help explain this finding (eg, awareness of the symptom status of family members may make sufferers more open to discussing their symptoms and seeking help for the condition).[50] Preliminary findings also suggest that IBS may be associated with select gene polymorphisms, including those in IL-10, G-protein GNb3, alpha adrenoceptor, and serotonin reuptake transporter (SERT).[47,51–54] Despite these potential links, however, conclusive evidence for a genetic basis for IBS has not been established.
Postinfectious IBS
The presence of postinfectious (PI)-IBS, referring to the development of IBS symptoms – particularly abdominal pain and diarrhea – shortly after an enteric infection, is based on research from prospective studies in which IBS symptoms developed in 7% to 32% of patients after they recovered from bacterial gastroenteritis.[52,55,56] Specific risk factors for the development of PI-IBS have been identified, including younger age, female sex, presence of severe infectious gastroenteritis for a prolonged period, use of antibiotics to treat this infection, and presence of concomitant psychological disorders (eg, anxiety).[39,52,55,57] Difficulty in downregulating intestinal inflammation in the colonic mucosa has been suggested as a potential underlying mechanism in this condition.[52] Also suggested as a potential underlying mechanism is the presence of colonic changes shown in patients with PI-IBS compared with controls, including increased gut permeability, increased mucosal enterochromaffin cell production, and increased concentration of mast cells and T lymphocytes in the gut mucosa.[39,52,55,57] Despite considerable evidence linking IBS with an inflammatory etiology (perhaps triggered by enteric infection), in a controlled trial of patients with PI-IBS, anti-inflammatory treatment with prednisolone was not more effective than placebo in improving patient symptoms.[58] The true role of prior infection as a key factor in PI-IBS remains to be established.[59]
The use of probiotics (products containing live or attenuated bacteria that have a positive effect on the host) in alleviating symptoms in patients with PI-IBS is an area of recent focus.[60,61] The potential utility of probiotics in this setting stems from their antibacterial, antiviral, and immune-modulating properties; their ability to modify intestinal flora; and their potential to enhance intestinal mucus secretion or influence stool consistency or volume and gas handling.[60] The number of studies evaluating the efficacy of probiotic preparations in patients with IBS is limited but growing.[60–68] Because trials vary in study design, dose, and strain (Lactobacillus and Bifidobacteria alone or in combination; mixture of Lactobacillus, Bifidobacteria, and Streptococcus), direct comparison of results is challenging. Overall, some degree of IBS symptom improvement has been demonstrated in symptoms such as abdominal pain,[65,66] bloating,[63,66] gas,[66] and daily symptom scores.[62,65] O'Mahoney and colleagues[60] have recently demonstrated that results with the Bifidobacterium infantis strain are particularly promising. In a separate analysis, these investigators showed that the baseline characteristics of urgency and hard stool increased the odds ratio of response to this strain, whereas straining and alcohol consumption reduced the likelihood of response.[69,70] The ultimate place in therapy of probiotics in IBS remains to be elucidated.
Small Intestinal Bacterial Overgrowth
The presence of a higher than usual population of bacteria in the small intestine (leading to bacterial fermentation of poorly digestible starches and subsequent gas production) has been proposed as a potential etiologic factor in IBS.[71] Pimentel and colleagues have shown that, when measured by the lactose hydrogen breath test (LHBT), small intestinal bacterial overgrowth (SIBO) has been detected in 78% to 84% of patients with IBS.[71,72] However, the accuracy of the LHBT in testing for the presence of SIBO has been questioned.[73] Sensitivity of the LHBT for SIBO has been shown to be as low as 16.7%, and specificity approximately 70%.[74] Additionally, this test may suboptimally assess treatment response.[75] The glucose breath test has been shown to be a more reliable tool,[76] with a 75% sensitivity for SIBO[77] vs 39% with LHBT for the “double-peak” method of SIBO detection.[74] In a recently conducted retrospective study involving review of patient charts for the presence of gastrointestinal-related symptoms (including IBS) in patients who were referred for glucose hydrogen breath tests for SIBO, of 113 patients who met Rome II criteria for IBS, 11% tested positive for SIBO.[78] Thus, results demonstrated that IBS symptoms are often unrelated to the presence of SIBO. Despite the controversy regarding the contribution of SIBO to the underlying pathophysiology of IBS and its symptoms, short-term placebo-controlled clinical studies with select antibiotics, including neomycin and rifaximin, have demonstrated symptom improvement in IBS patients.[61,72,79] Antibiotics may therefore have potential utility in select subgroups of IBS patients in whom SIBO contributes to symptoms. However, the chronic nature of IBS symptoms often leads to the need for long-term treatment. Given the fact that long-term use of antibiotics is generally undesirable, the place of antibiotics in IBS therapy remains to be established.[73]
Food Intolerance
Food intolerance has been proposed as a potential cause of GI symptoms in some patients with IBS; however, this link is not well established. Although some patients associate onset of IBS symptoms with ingestion of particular foods, identification of a true food intolerance is challenging, and elimination diets are typically time-consuming and difficult to implement. Recent research involving exclusion of foods to which patients had immunoglobulin (Ig) G antibodies, which are associated with a more delayed response after antigen exposure than IgE antibodies, resulted in significantly better symptom improvement than in patients in the nonexclusion group.[80] Further research into the role of food intolerance in IBS is warranted.
Serotonin Signaling
Of the putative mechanisms underlying the pathophysiology of IBS, the strongest evidence points to the role of serotonin in the GI tract. The effect of serotonergic mechanisms in the manifestation of IBS symptoms has led to development of a new drug class for the treatment of IBS patients: the GI serotonergic agents.
Normal GI function relies on a properly functioning brain-gut axis, which involves the coordinated interplay of the GI musculature, the CNS, the autonomic nervous system, and the enteric nervous system (ENS). The ENS contains millions of neurons embedded in the wall of the digestive tract and functions, at least in part, independently of the CNS. The size, complexity, and independent function of the ENS has resulted in application of the terms “the second brain” and “the mini-brain.”[81] Impaired function or coordination of any of these systems, or the communication between these systems and the GI musculature, can lead to symptoms of dysmotility and altered sensory perception, which are characteristic of IBS and select other GI motility disorders.[82]
The neurotransmitter serotonin (5-hydroxytryptamine [5-HT]) is a predominant signaling molecule in the ENS. Most (90% to 95%) of the body's serotonin is found in the gut, and smaller amounts are found in the brain (about 3%) and in platelets (about 2%).[83] In the GI tract, serotonin facilitates communication between the ENS and its effector systems (muscles, secretory endothelium, endocrine cells, and vasculature of the GI tract), thus playing a key role in normal GI tract functioning.[84] In addition, serotonin plays a role in the communication between the ENS and the CNS.
In the gut, serotonin is synthesized by and stored in the enterochromaffin cells, which are located within the mucosa of the intestinal wall. When material passes through the lumen and the mucosa is stimulated, enterochromaffin cells release serotonin, which then binds to its receptors (primarily 5-HT1P receptors) on intrinsic primary afferent neurons, initiating peristalsis and secretion. Serotonin also binds to 5-HT4 receptors on interneurons, which augments the transmission of signals to motor neurons, resulting in enhanced peristaltic activity. In transgenic mice lacking 5-HT4 receptors, colonic motility is abnormally slow, confirming the role of these receptors in facilitating normal colonic motility.[85] By binding to 5-HT3 receptors on efferent sensory innervations coming from the vagus and the spinal nerves, serotonin mediates signaling between the ENS and the CNS and, thus, modulates pain perception.
To regulate the signaling process, excess serotonin must be removed; this is accomplished by the SERT molecule expressed by intestinal epithelial cells.[86] Human studies have shown that defects in serotonin signaling contribute to the pathophysiology of IBS and, potentially, other GI motility disorders. In a recent study by Coates and colleagues,[87] biopsy specimens from patients with IBS showed significantly lower mucosal serotonin concentrations than those from healthy controls, potentially the result of lower mRNA levels for tryptophan hydroxylase (the rate-limiting enzyme in serotonin synthesis), which were also significantly lower in patients with inflammatory bowel disease.[87] There was no significant difference in the number of enterochromaffin cells or in the capacity of these cells to release serotonin under stimulated conditions. In another study, higher serotonin levels were observed in mucosal biopsy samples from patients with IBS with constipation (IBS-C) than in patients with IBS-D or in healthy volunteers.[88]
Serotonin levels may also be affected by altering the amount or function of SERT. The study by Coates and colleagues[87] showed a significant decrease in the level of SERT mRNA and SERT protein expressed in the intestinal epithelial cells of IBS patients compared with that of healthy volunteers. In another study,[89] SERT expression and binding capacity in platelets were decreased in women with IBS-D compared with expression and binding capacity in healthy controls. Furthermore, Chen and colleagues[90] showed that mice with a SERT gene deletion had altered colonic motility. It is interesting to note that the mice thrived in laboratory housing conditions, indicating that other transporters could compensate for the lack of SERT. Additional studies have focused on SERT polymorphisms. Yeo and colleagues[91] showed an association between patients with IBS-D and the homozygous short polymorphism of the SERT gene promoter. This mutation results in lower levels of SERT gene transcription and reduced amounts of SERT protein available for reuptake of serotonin. In addition, Camilleri and colleagues[92] showed a possible link between the long promoter polymorphism and patient response to therapy.
Thus, a substantially large body of work shows that normal gut physiology is predicated on the interplay between the GI musculature and the ENS, autonomic nervous system, and CNS. One of the central mediators of this complex interplay is the neurotransmitter serotonin. Impairment or imbalance in serotonergic signaling, which can affect GI motility, secretion, and visceral sensitivity, may be affected by defects or deficiencies in serotonin production, specific serotonin receptors, or proteins such as SERT. These changes can manifest in symptoms associated with IBS, including abdominal pain, altered bowel habits (constipation, diarrhea, or alternation between these 2 states), and bloating.
IBS Treatments
Traditional Treatment Options
The goal of IBS treatment is to provide relief of symptoms and improve overall well-being.[2] Traditional therapies for IBS can effectively treat single symptoms (Table 1), but their utility may be limited when patients have moderate-to-severe symptoms or when they have multiple symptoms. The multisymptom nature of IBS often leads patients to try numerous treatment methods to achieve a broad spectrum of symptom relief. Additionally, many of these agents are associated with adverse effects that may limit their routine use. Some of these adverse effects mimic IBS symptoms (eg, fiber may cause bloating, tricyclic antidepressants may cause constipation).[2]
Table 1.
Pharmacologic Therapies for IBS Primarily Target Single Symptoms
| Drug Category | Examples | Therapeutic Rationale | Primary Target Symptoms | Tolerability Concerns |
|---|---|---|---|---|
| Traditional Therapies | ||||
| Bulking agents[2] | Wheat bran, corn fiber, calcium polycarbophil, psyllium | Accelerate intestinal transit, add fluid to stool mass, and create gel-like matrix in stool | Constipation, diarrhea | May increase intestinal gas, bloating, and abdominal discomfort |
| Osmotic laxatives[137] | Magnesium hydroxide, sodium phosphate, lactulose, sorbitol solution | Poorly absorbed ions or sugars that cause an influx of fluid and electrolytes into the intestine | Constipation | Diarrhea, dehydration, electrolyte disorders |
| Stimulant laxatives[137] | Senna, bisacodyl, castor oil, aloe | Reduce water and electrolyte absorption by stimulating colonic neurons and irritating the colonic mucosa | Constipation | Dehydration, electrolyte disturbances, significant cramping, and diarrhea. Should be avoided |
| Antidiarrheal agents[2] | Loperamide | Delay intestinal transit and may enhance resting internal anal sphincter tone | Diarrhea | May cause constipation: should not be used in patients with IBS-C; use with caution in patients with alternating IBS symptoms |
| Antispasmodic agents[2] | Hyoscyamine, dicyclomine | Anticholinergic effects and decreased spontaneous activity of intestinal smooth muscle | Diarrhea, abdominal pain | Anticholinergic adverse effects at high doses (including urinary retention and constipation). Use with caution in patients with IBS-C |
| Decreased fluid and electrolyte secretion | ||||
| Antidepressants[2,138] | Desipramine, amitriptyline, trimipramine, doxepin, paroxetine, fluoxetine | Decreased gut sensitivity, decreasing experience of abdominal pain | Pain | Constipation, dry mouth, and dizziness are common adverse effects of TCAs |
| Antianxiety and antidepressant effects | Diarrhea, nausea, nervousness, and fatigue are common adverse effects of SSRIs | |||
| Multiple-Symptom Relief | ||||
| 5-HT3 receptor antagonists (methylimidazole analogs)[102] | Alosetron | Reduce visceral sensitivity and colonic transit | Abdominal pain, diarrhea, urgency | Alosetron: Black box warning regarding serious consequences of constipation; ischemic colitis (reported during clinical trials and in postmarketing surveillance) |
| 5-HT4 receptor agonists[111] (aminoguanidine indoles) | Tegaserod | Reduce visceral sensitivity and increase GI transit and intestinal secretion | Abdominal pain/discomfort, bloating, constipation | Diarrhea, ischemic colitis (reported in postmarketing surveillance) |
IBS = irritable bowel syndrome; IBS-C = IBS with constipation; TCA = tricyclic antidepressant; SSRI = selective serotonin reuptake inhibitor.
Because of their ability to alter serotonin function in the CNS and possibly in the periphery, low-dose tricyclic antidepressants and selective serotonin reuptake inhibitors are sometimes used to treat patients with IBS. They are primarily effective in reducing the pain associated with this disorder and are used at doses well below those used for psychiatric indications such as depression.[2,3] Although evidence suggests that some patients with IBS may benefit from tricyclic antidepressants or selective serotonin reuptake inhibitor therapy, results of clinical studies show significant variability in the response and do not provide overwhelming support for their use, except perhaps in patients with comorbid psychiatric conditions and a lack of response to other therapies.[93,94]
Along with traditional pharmacologic therapies, additional measures such as patient education and behavioral therapy (eg, hypnosis, biofeedback, relaxation, cognitive therapy) are used in treating patients with IBS. Most studies use a combination of these techniques. Biofeedback is one example of a noninvasive, behavioral intervention that may help patients with IBS achieve symptom relief. In this approach, sensors are strategically placed at specific positions on patients, and a computer and video monitor are used to illustrate (via sounds or line graphs) body functions of which patients are normally not aware. This information can be used to help patients recognize and modify (eg, increase, decrease, coordinate) their physiologic responses to various stressful stimuli (eg, stress-related motility).[95,96] The value of biofeedback and other behavioral therapies cannot be discounted in the management strategy for IBS and should be considered in the overall treatment plan.[2] A recently reported systematic review of published trials of psychological treatments for IBS, including biofeedback, found an overall positive outcome for these interventions (odds ratio of treatment effect [50% reduction in symptoms] of 12 [95% CI, 5.56-25.96]); however, this study did not examine the comparative efficacy of one psychological treatment over another.[97] To maximize the likelihood of success with behavioral therapy, these techniques should be administered by a trained professional (eg, physician, physical or occupational therapist, nurse). The scarcity of such trained personnel often limits the utility of these approaches.[95,96]
US Food and Drug Administration-Approved Gastrointestinal Serotonergic Agents
As discussed previously, increased understanding of the role of serotonin in gut physiology has led to the development of drugs designed to modulate serotonin function in the GI tract. Serotonin affects GI motility, secretion, and visceral sensitivity – all of which are altered in patients with IBS. Drugs designed to target key serotonin receptors in the GI tract, including 5-HT3 receptor antagonists (eg, alosetron for IBS-D) and 5-HT4 receptor agonists (eg, tegaserod for IBS-C), effectively improve multiple symptoms of IBS. These agents have been tested in large, randomized, placebo-controlled trials and were found to be more effective than placebo in providing global relief of IBS symptoms. Alosetron and tegaserod received grade A recommendations (the highest possible) from the ACG Functional GI Disorders Task Force in its systematic review of treatments for IBS.[2]
Alosetron
Alosetron is a 5-HT3 receptor antagonist originally approved by the US Food and Drug Administration (FDA) in 2000 for the treatment of women with IBS-D. In large, double-blind, placebo-controlled clinical trials, alosetron (1 mg twice daily) was significantly more effective than placebo in improving individual symptoms (eg, bowel urgency, abdominal pain) of IBS-D,[98,99] as well as in global symptom improvement (as measured using the IBS Global Improvement Scale).[100,101] The most frequently reported adverse effects during clinical trials, for alosetron and placebo, respectively, were constipation (29% vs 6%), abdominal pain/discomfort (7% vs 4%), and nausea (6% vs 5%).[102] Constipation generally occurred as single episodes during the first month of treatment and resolved either on its own or after an interruption in treatment.[102] Ischemic colitis and serious complications related to constipation were reported during the clinical trials and during postmarketing surveillance, prompting the withdrawal of the drug from the market less than a year after its introduction. Toward the end of 2002, however, alosetron was reintroduced with a restricted indication for women with severe IBS-D who have not responded to conventional therapy and in whom anatomic and biochemical abnormalities have been excluded.[102] Furthermore, the new prescribing label (revised again in February 2005) includes: (1) a black box warning regarding the risk for serious GI adverse effects, such as ischemic colitis; (2) a new starting dosage (0.5 mg twice daily); and (3) a requirement that physicians and patients subscribe to a risk-management program. The risk-management program was put into place to ensure that physicians and patients are aware of the potential for serious adverse effects and agree to follow steps to maximize the safe use of alosetron.
Recently, the long-term efficacy and safety profile of alosetron (1 mg twice daily) was shown in a 12-month randomized, double-blind, placebo-controlled trial in women with severe IBS-D.[103] In this trial, constipation occurred in 23% of patients taking alosetron and in 5% of those receiving placebo; however, no cases of ischemic colitis or serious constipation were documented.[103] Current clinical trials (phase 2) are aimed specifically at showing the efficacy and safety of alosetron in men with IBS-D.[104]
Tegaserod
Tegaserod is a selective 5-HT4 receptor agonist approved by the FDA for the short-term treatment of women with IBS-C and for men and women younger than 65 years of age with chronic idiopathic constipation. In large, randomized, placebo-controlled clinical trials, tegaserod (6 mg twice daily) provided significantly greater global relief of IBS symptoms, as assessed using the Subject's Global Assessment of Relief, and relief of individual IBS-C symptoms (eg, stool consistency and frequency, abdominal pain and discomfort, bloating).[105–108] Treatment response was observed within the first week of therapy and continued for the 12-week duration of the trials. Additional studies, in which tegaserod was stopped until symptoms returned and then restarted, indicate that symptoms recur in most patients after tegaserod is withheld, and re-treatment yields response rates similar to those observed during initial treatment.[109–110]
The adverse effects occurring significantly more frequently in tegaserod-treated vs placebo-treated patients, respectively, in IBS clinical trials were headache (15% and 12%) and diarrhea (9% and 4%).[111] In most cases, diarrhea was mild, occurred as a single episode during the first week of therapy, and resolved with continued treatment. Discontinuation of the use of tegaserod as a result of diarrhea was low (1.6% vs 0% for those receiving placebo). Clinically significant diarrhea (resulting in hospital admission, hypovolemia, hypotension, and need for intravenous fluid) occurred in a minority (4 [0.04%] of 10,000) of patients in clinical trials.[111] A long-term, open-label safety study showed that tegaserod was safe and well tolerated in patients receiving 6 mg twice daily for up to 1 year.[112]
Emerging Treatments for IBS
The enhanced understanding of putative underlying mechanisms in IBS has led the way for the development of innovative drug classes. Numerous agents are in the drug development pipeline, including newer GI serotonergic agents, alpha2-adrenergic agonists, kappa-opioid receptor agonists, chloride channel openers, 2,3 benzodiazepines, and guanylate cyclase-C agonists. A brief overview of the mechanism of action and potential place in IBS therapy of some of these novel agents appears and is summarized in Table 2.
Table 2.
Emerging Pharmacologic Classes for IBS
| Drug Class | Examples | Mechanism of Action | Potential Utility/Place in Therapy | Limitations/Comments | |
|---|---|---|---|---|---|
| Newer GI serotonergic agents | Cilansetron (5-HT3 receptor antagonist)[113–115] | Inhibits colonic motility and reduces visceral hypersensitivity | IBS-D | Constipation is a common adverse effect | |
| Cases of ischemic colitis have been reported (all resolved with complications) within several weeks | |||||
| Currently in phase e3 clinical trials. FDA approval was rejected in April 2005 | |||||
| Renzapride (mixed 5-HT4 receptor agonists/5-HT3 receptor antagonist [benzamide derivative])[117–120] | Accelerates colonic transit and reduces visceral hypersensitivity | IBS-C | In phase 2 clinical trials, diarrhea and headache were frequently reported adverse effects | ||
| Alpha2-Adrenergic agonist | Clonidine[118-120,139] | Reduces colonic motor activity and pain sensitivity | IBS-D | Commonly reported adverse effects include somnolence and hypotension | |
| kappa-Opioid receptor agonists | Asimadoline[122,123] | Reduces colonic sensation without affecting GI motility | Has been evaluated in a preliminary study in patients with IBS-C | Does not cross the blood-brain barrier (and is therefore unlikely to cause central side effects typical of opioid agonists, including constipation) | |
| Chloride Channel Openers | Lubiprostone*[127,128] | Increases intestinal fluid secretion, thereby softening the stools, promoting bowel movement, and decreasing bloating and abdominal pain | IBS-C | In phase III clinical trials of patients with constipation, the most commonly reported adverse effects were headache, diarrhea, and nausea | |
| A phase III clinical trial in patients with IBS-C began in mid-2005 | |||||
| 2,3 Benzodiazepines | Dextofisopam[131–133] | Decreases autonomic function by binding to 2,3 benzodiazepine receptors | IBS-C and IBS-A | In a Phase 2 clinical trial, abdominal pain occurred more frequently in the dextofisopam treatment group, whereas headache was more common in the placebo group | |
| Guanylate cyclase-C agonist | MD-1100[134–136] | Act locally on intestinal epithelium to enhance secretion and motility and reduce visceral sensation. | IBS-C | Phase 1 study found MD-100 to be well tolerated. No systemic absorption was detected, and a decrease in stool consistency (assessed by the Bristol Stool Scale) was noted. | A Phase II study with patients with IBS-C is underway. |
| A Phase II study with patients with IBS-C is underway. |
GI = gastrointestinal; IBS = irritable bowel syndrome; IBS-C = IBS with constipation; IBS-D = IBS with diarrhea.
Recently FDA-approved for the treatment of patients with chronic idiopathic constipation.
Cilansetron is a 5-HT3 receptor antagonist.[113–116] Similar to alosetron, cilansetron works by inhibiting colonic motility and reducing visceral hypersensitivity. This agent has been shown to be effective (in both male and female patients) in relieving abnormal bowel habits and abdominal pain in patients with IBS-D. Constipation is a common adverse effect. Cases of ischemic colitis have been reported, but all resolved (without complications) within several weeks. This agent received a nonapproval letter from the FDA in April of 2005.
Renzapride is a mixed 5-HT4 receptor agonists/5-HT3 receptor antagonist (benzamide derivative)[117–120] that works by accelerating colonic transit and reducing visceral hypersensitivity. Preliminary data demonstrate a beneficial effect of renzapride in improving symptoms of abdominal pain/discomfort, stool consistency, and stool frequency in patients with IBS-C; its utility in patients with alternating bowel habits (IBS-A) is less clear, however. In phase 2 clinical trials, diarrhea and headache were frequently reported adverse effects.[117–120]
Clonidine is an alpha2-adrenergic agonist that works by reducing colonic motor activity and pain sensitivity. In a study conducted in patients with IBS-D, clonidine reduced bowel dysfunction and enhanced the proportion of patients achieving satisfactory relief of IBS, without altering gastrointestinal transit.[121] Commonly reported adverse effects, including somnolence and hypotension, may limit its usefulness on a chronic basis.
Asimadoline[122,123] is a kappa-opioid receptor agonist that exerts anti-nociceptive properties on the gut by reducing colonic sensation without affecting gastrointestinal motility. This agent does not cross the blood-brain barrier and therefore works in a peripheral manner (partially though blockage of gut sodium channels).[124,125] Its efficacy in reducing sensitivity to colonic distension (without altering compliance or tone) has been shown in a preliminary study in patients with IBS-C.[126]
Lubiprostone (a prostaglandin E1 derivative bicyclic fatty acid) acts as a chloride channel opener, increasing intestinal fluid secretion and thereby softening stools, promoting bowel movement, and decreasing bloating and abdominal pain.[127] In phase 3 clinical trials of patients with constipation, the most commonly reported adverse effects were headache, diarrhea, and nausea.[128] A phase 3 clinical trial in patients with IBS-C was initiated in mid-2005.[127]
Dextofisopam is classified as a 2,3 benzodiazepine. Unlike typical benzodiazepines, this agent does not bind to GABA receptors, but rather to 2,3 benzodiazepine receptors.[129,130] Although opioid neurotransmission and altered phosphorylation of proteins that affect signal transmission are among the proposed modes of action, the exact mechanism by which this drug class works continues to be evaluated.[129] The efficacy of dextofisopam in male and female patients with IBS-D or IBS-A was demonstrated in a randomized, placebo-controlled, phase 2b 12-week study. For the primary efficacy endpoint (overall IBS symptom relief), dextofisopam was more effective than placebo for both IBS subtypes. For the secondary endpoints of stool frequency and stool consistency, however, dextofisopam was more effective vs placebo in patients with IBS-D than those with IBS-A.[131–133] Abdominal pain occurred more frequently in the dextofisopam treatment groups, whereas headache occurred more often in the placebo groups.[131–133]
MD-1100 is an agonist of guanylate cyclase-C, a receptor located on intestinal cell surfaces. In preclinical studies, this compound has been shown to act locally on the intestinal epithelium to enhance secretion and motility and reduce visceral sensation. This agent is in early-stage development for the treatment of patients with IBS-C. Results from a double-blind, randomized, placebo-controlled, single-dose phase 1 study found MD-1100 to be well tolerated. No systemic absorption was detected, and a decrease in stool consistency (as assessed by the Bristol Stool Scale) was noted.[134,135] A multiple, ascending-dose phase 1 study is ongoing, and a phase II study with patients with IBS-C is under way.[135,136]
Conclusion
Advances in research over the past several decades have paved the way for an enhanced understanding of the underlying pathophysiology of IBS; development of standardized symptom-based approaches that can be implemented in making a positive diagnosis; and development of innovative treatment options that target multiple IBS symptoms. Although many unanswered questions remain, the progress is promising and it has equipped physicians better to efficiently diagnose IBS and choose from a growing armamentarium of treatment options.
References
- 1.American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S1–S5. doi: 10.1016/s0002-9270(02)05656-3. [DOI] [PubMed] [Google Scholar]
- 2.Brandt LJ, Bjorkman D, Fennerty MB, et al. Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S7–S26. doi: 10.1016/s0002-9270(02)05657-5. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 4.Hungin APS, Tack J, Mearin F, Whorwell PJ, Dennis E, Barghout V. Irritable bowel syndrome (IBS): prevalence and impact in the USA – the truth in IBS (T-IBS) survey [abstract] Am J Gastroenterol. 2002;97:S280–S281. [Google Scholar]
- 5.Glia A, Lindberg G. Quality of life in patients with different types of functional constipation. Scand J Gastroenterol. 1997;32:1083–1089. doi: 10.3109/00365529709002985. [DOI] [PubMed] [Google Scholar]
- 6.Dean BB, Aguilar D, Barghout V, et al. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11:S17–S26. [PubMed] [Google Scholar]
- 7.Whitehead WE, Burnett CK, Cook EW, III, Taub E. Impact of irritable bowel syndrome on quality of life. Dig Dis Sci. 1996;41:2248–2253. doi: 10.1007/BF02071408. [DOI] [PubMed] [Google Scholar]
- 8.El Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16:1171–1185. doi: 10.1046/j.1365-2036.2002.01290.x. [DOI] [PubMed] [Google Scholar]
- 9.Frank L, Kleinman L, Rentz A, Ciesla G, Kim JJ, Zacker C. Health-related quality of life associated with irritable bowel syndrome: comparison with other chronic diseases. Clin Ther. 2002;24:675–689. doi: 10.1016/s0149-2918(02)85143-8. [DOI] [PubMed] [Google Scholar]
- 10.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654–660. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 11.Martin R, Barron JJ, Zacker C. Irritable bowel syndrome: toward a cost-effective management approach. Am J Manag Care. 2001;7:S268–S275. [PubMed] [Google Scholar]
- 12.American Gastroenterological Association. The Burden of Gastrointestinal Diseases. Bethesda, MD: American Gastroenterological Association; 2001. pp. 1–86. Available at: http://www.gastro.org/clinicalRes/pdf/burden-report.pdf. Accessed November 16, 2004. [Google Scholar]
- 13.Camilleri M, Williams DE. Economic burden of irritable bowel syndrome. Proposed strategies to control expenditures. PharmacoEconomics. 2000;17:331–338. doi: 10.2165/00019053-200017040-00003. [DOI] [PubMed] [Google Scholar]
- 14.Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109:1736–1741. doi: 10.1016/0016-5085(95)90738-6. [DOI] [PubMed] [Google Scholar]
- 15.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(suppl II):II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. BMJ. 1978;2:653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lembo TJ, Fink RN. Clinical assessment of irritable bowel syndrome. J Clin Gastroenterol. 2002;35:S31–S36. doi: 10.1097/00004836-200207001-00007. [DOI] [PubMed] [Google Scholar]
- 18.Talley NJ, Phillips SF, Melton LJ, Mulvihill C, Wiltgen C, Zinsmeister AR. Diagnostic value of the Manning criteria in irritable bowel syndrome. Gut. 1990;31:77–81. doi: 10.1136/gut.31.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson WG, Creed FH, Drossman DA. Functional bowel disorders and functional abdominal pain. Gastroenterol Int. 1992;5:75–91. [Google Scholar]
- 20.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Longstreth GF, Thompson G, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 22.Whitehead WE, Palsson O, Levy R, et al. Agreement of rome criteria with clinical diagnosis of irritable bowel (IBS) [abstract] Gastroenterology. 2003;124:A–397. [Google Scholar]
- 23.Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol. 1999;94:2912–2917. doi: 10.1111/j.1572-0241.1999.01437.x. [DOI] [PubMed] [Google Scholar]
- 24.Kwan AC-P, Bao TN, Chakkaphak S, et al. Validation of Rome II criteria for functional gastrointestinal disorders by factor analysis of symptoms in Asian patient sample. J Gastroenterol Hepatol. 2003;18:796–802. doi: 10.1046/j.1440-1746.2003.03081.x. [DOI] [PubMed] [Google Scholar]
- 25.Chey WD, Olden K, Carter E, Boyle J, Drossman D, Chang L. Utility of the Rome I and Rome II criteria for irritable bowel syndrome in U.S. women. Am J Gastroenterol. 2002;97:2803–2811. doi: 10.1111/j.1572-0241.2002.07026.x. [DOI] [PubMed] [Google Scholar]
- 26.Mearin F, Badia X the IBER Group. How restrictive are Rome II criteria for the diagnosis of irritable bowel syndrome (IBS) [abstract]? Gut. 2000;47:A215. [Google Scholar]
- 27.Thompson GW, Irvine JE, Pare P, Ferrazzi S, Rance L. Comparing Rome I and Rome II criteria for irritable bowel syndrome (IBS) in a prospective survey of the Canadian population [abstract] Am J Gastroenterol. 2000;95:2553. [Google Scholar]
- 28.Williams RE, Black CL, Kim HY, et al. Stability of irritable bowel syndrome using a Rome II-based classification. Aliment Pharmacol Ther. 2006;23:197–205. doi: 10.1111/j.1365-2036.2006.02723.x. [DOI] [PubMed] [Google Scholar]
- 29.Zanten SV. Diagnosing irritable bowel syndrome. Rev Gastroenterol Dis. 2003;3:S12–S17. [PubMed] [Google Scholar]
- 30.Paterson WG, Thompson WG, Vanner SJ, et al. Recommendations for the management of irritable bowel syndrome in family practice. IBS Consensus Conference Participants. CMAJ. 1999;161:154–160. [PMC free article] [PubMed] [Google Scholar]
- 31.Camilleri M, Choi MG. Review article: irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:3–15. doi: 10.1046/j.1365-2036.1997.84256000.x. [DOI] [PubMed] [Google Scholar]
- 32.Cash BD, Schoenfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol. 2002;97:2812–2819. doi: 10.1111/j.1572-0241.2002.07027.x. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel BM, Derosa VP, Gralnek IM, Wang V, Dulai GS. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: A cost-effectiveness analysis. Gastroenterology. 2004;126:1721–1732. doi: 10.1053/j.gastro.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Hamm LR, Sorrells SC, Harding JP, et al. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol. 1999;94:1279–1282. doi: 10.1111/j.1572-0241.1999.01077.x. [DOI] [PubMed] [Google Scholar]
- 35.Tolliver BA, Herrera JL, DiPalma JA. Evaluation of patients who meet clinical criteria for irritable bowel syndrome. Am J Gastroenterol. 1994;89:176–178. [PubMed] [Google Scholar]
- 36.Talley NJ. When to conduct testing in patients with suspected irritable bowel syndrome. Rev Gastroenterol Disord. 2003;3:S18–S24. [PubMed] [Google Scholar]
- 37.Adeniji OA, Barnett CB, Di Palma JA. Durability of the diagnosis of irritable bowel syndrome based on clinical criteria. Dig Dis Sci. 2004;49:572–574. doi: 10.1023/b:ddas.0000026300.47363.3b. [DOI] [PubMed] [Google Scholar]
- 38.Locke GR., III Natural history of irritable bowel syndrome and durability of the diagnosis. Rev Gastroenterol Dis. 2003;3:S12–S17. [PubMed] [Google Scholar]
- 39.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Salvioli B, Corinaldesi R. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:1–9. doi: 10.1111/j.1365-2036.2004.02036.x. [DOI] [PubMed] [Google Scholar]
- 40.Drossman DA. What does the future hold for irritable bowel syndrome and the functional gastrointestinal disorders? J Clin Gastroenterol. 2005;39:S251–S256. doi: 10.1097/01.mcg.0000156107.13247.69. [DOI] [PubMed] [Google Scholar]
- 41.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 42.Drossman DA. Gastrointestinal illness and the biopsychosocial model [editorial] J Clin Gastroenterol. 1996;22:252–254. doi: 10.1097/00004836-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Drossman DA. Presidential address: gastrointestinal illness and the biopsychosocial model. Psychosom Med. 1998;60:258–267. doi: 10.1097/00006842-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Eutamene H, Theodorou V, Fioramonti J, Bueno L. Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin-1 and corticotropin-releasing factor release in rats. J Physiol Lond. 2003;553:959–966. doi: 10.1113/jphysiol.2003.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tache Y, Monnikes H, Bonaz B, Rivier J. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann NY Acad Sci. 1993;697:233–243. doi: 10.1111/j.1749-6632.1993.tb49936.x. [DOI] [PubMed] [Google Scholar]
- 46.Sagami Y, Shimada Y, Tayama J, et al. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falk P. Is IBS a genetic disorder? FBG Newsletter. 2003:10–15. [Google Scholar]
- 48.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 49.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 50.Locke GR, III, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ., III Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75:907–912. doi: 10.4065/75.9.907. [DOI] [PubMed] [Google Scholar]
- 51.Camilleri M. Is there a SERT-ain association with IBS? Gut. 2004;53:1396–1399. doi: 10.1136/gut.2004.039826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51(suppl 1):i41–i44. doi: 10.1136/gut.51.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HJ, Camilleri M, Carlson PJ, et al. Association of distinct alpha(2) adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut. 2004;53:829–837. doi: 10.1136/gut.2003.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holtmann G, Siffert W, Haag S, et al. G-protein beta3 subunit 825 CC genotype is associated with unexplained (functional) dyspepsia. Gastroenterology. 2004;126:971–979. doi: 10.1053/j.gastro.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Mach T. The brain-gut axis in irritable bowel syndrome–clinical aspects. Med Sci Monit. 2004;10:RA125–RA131. [PubMed] [Google Scholar]
- 56.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 58.Dunlop S, Jenkins D, Neal KR, et al. Randomized, double-blind, placebo-controlled trial of Prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:77–84. doi: 10.1046/j.1365-2036.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 59.Camilleri M. Mechanisms in IBS: something old, something new, something borrowed. Neurogastroenterol Motil. 2005;17:311–316. doi: 10.1111/j.1365-2982.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- 60.O'Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 61.Harris LA, Chang L. Irritable bowel syndrome: new and emerging therapies. Curr Opin Gastroenterol. 2006;22:128–135. doi: 10.1097/01.mog.0000208461.84513.f3. [DOI] [PubMed] [Google Scholar]
- 62.Halpern GM, Prindiville T, Blankenburg M, et al. Treatment of irritable bowel syndrome with Lacteol Fort: a randomized, double-blind, cross-over trial. Am J Gastroenterol. 1996;91:1579–1585. [PubMed] [Google Scholar]
- 63.Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic: VSL#3, on gut transit and symptoms in diarrhea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 64.Niv E, Naftali T, Hallak R, Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome: a double blind, placebo-controlled, randomized study. Clin Nutr. 2005;24:925. doi: 10.1016/j.clnu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Niedzielin K, Kordecki H, Birkenfield B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus planetarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–1147. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Noback S, Johansson ML, Molin G, et al. Alterations in intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 67.O'Sullivan MA, O'Morain CA. Bacterial supplementation in the irritable bowel syndrome: a randomized double-blind placebo-controlled cross-over study. Dig Liver Dis. 2000;32:294–301. doi: 10.1016/s1590-8658(00)80021-3. [DOI] [PubMed] [Google Scholar]
- 68.Sen S, Mullan MM, Parker TJ, et al. Effect of Lactobacillus plantarum 290v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47:2615–2620. doi: 10.1023/a:1020597001460. [DOI] [PubMed] [Google Scholar]
- 69.Quigley EM, Whorwell PJ, Altringer L, Morel J, O'Mahony L, Shanahan F. Who is the responder to probiotic therapy in IBS? Data from a controlled clinical trial with Bifidobacterium infantis 35624. Am J Gastroenterol. 2005;100:S325–S326. doi: 10.1111/j.1572-0241.2006.00734.x. [Abstract #887] [DOI] [PubMed] [Google Scholar]
- 70.Lin Chang MD. New updates in chronic constipation and irritable bowel syndrome. Medscape Gastroenterology. 2005 http://www.medscape.com/viewarticle/517739 Accessed May 25, 2006. [Google Scholar]
- 71.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 72.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 73.Parisi G, Leandro G, Bottona E, et al. Small intestinal bacterial overgrowth and irritable bowel syndrome. Am J Gastroenterol. 2003;98:2572–2574. doi: 10.1111/j.1572-0241.2003.08686.x. [DOI] [PubMed] [Google Scholar]
- 74.Riordan S, McIver C, Walker B, et al. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;9:1795–1803. [PubMed] [Google Scholar]
- 75.O'Leary C, Quigley EM. Small bowel bacterial overgrowth, celiac disease, and IBS: what are the real associations? Am J Gastroenterol. 2003;98:720–722. doi: 10.1111/j.1572-0241.2003.07395.x. [DOI] [PubMed] [Google Scholar]
- 76.Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982–988. doi: 10.1016/0016-5085(88)90173-4. [DOI] [PubMed] [Google Scholar]
- 77.Hamilton LH. Breath Tests and Gastroenterology. 2nd ed. Milwaukee, Wis: Quintron Instruments; 1998. Bacterial overgrowth. [Google Scholar]
- 78.Harris LA, Crowell MD, DiBaise JK, Olden K. Is small intestinal bacterial overgrowth (SIBO) really prevalent in irritable bowel syndrome (IBS)? Am J Gastroenterol. 2005;100:S336–S337. [Abstract #920] [Google Scholar]
- 79.Pimentel M, Park S, Kong Y, et al. Rifaximin, a non-absorbable antibiotic, improves the symptoms of irritable bowel syndrome: a double-blind randomized controlled study. Am J Gastroenterol. 2005;100:A–882. [Google Scholar]
- 80.Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459–1464. doi: 10.1136/gut.2003.037697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gershon MD. The enteric nervous system: a second brain. Hosp Pract (Off Ed) 1999;34:31–32. 35–38, 41–42. doi: 10.3810/hp.1999.07.153. [DOI] [PubMed] [Google Scholar]
- 82.Crowell MD. The role of serotonin in the pathophysiology of irritable bowel syndrome. Am J Manag Care. 2001;7:S252–S260. [PubMed] [Google Scholar]
- 83.Gershon MD. Serotonin and its implication for the management of irritable bowel syndrome. Rev Gastroenterol Disord. 2003;3:S25–S34. [PubMed] [Google Scholar]
- 84.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39:S184–S193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 85.Fiorica-Howells E, Liu MT, Ponimaskin EG, et al. Distribution of 5HT4 receptors in wild-type mice and analyses of intestinal motility in 5 HT4 knockout mice. Presented at: Digestive Diseases Week and the 104th Annual Meeting of the American Gastroenterological Association; May 17-22, 2003; Orlando, Florida. [Google Scholar]
- 86.Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol. 1998;275:G433–G448. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 87.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 88.Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–194. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- 89.Bellini M, Rappelli L, Blandizzi C, et al. Platelet serotonin transporter in patients with diarrhea-predominant irritable bowel syndrome both before and after treatment with alosetron. Am J Gastroenterol. 2003;98:2705–2711. doi: 10.1111/j.1572-0241.2003.08669.x. [DOI] [PubMed] [Google Scholar]
- 90.Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeo A, Boyd P, Lumsden S, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 93.Talley NJ. SSRIs in IBS: sensing a dash of disappointment. Clin Gastroenterol Hepatol. 2003;1:155–159. doi: 10.1053/cgh.2003.50023. [DOI] [PubMed] [Google Scholar]
- 94.Talley NJ. Antidepressants in IBS: are we deluding ourselves? Am J Gastroenterol. 2004;99:921–923. doi: 10.1111/j.1572-0241.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 95.Tries J, Plummer MK, Wisconsin OTR. Biofeedback and bowel disorders: Teaching yourself to live without the problem. Available at: http://www.aboutibs.org/Publications/Biofeedback.html Accessed May 26, 2006.
- 96.Irritable bowel syndrome. Available at: http://www.aapb.org/i4a/pages/index.cfm?pageid=3368 Accessed May 26, 2006.
- 97.Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol. 2004;72:1100–1113. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- 98.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 99.Jones RH, Holtmann G, Rodrigo L, et al. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13:1419–1427. doi: 10.1046/j.1365-2036.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- 100.Lembo T, Wright RA, Bagby B, et al. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:2662–2670. doi: 10.1111/j.1572-0241.2001.04128.x. [DOI] [PubMed] [Google Scholar]
- 101.Lembo AJ, Olden KW, Ameen VZ, Gordon SL, Heath AT, Carter EG. Effect of alosetron on bowel urgency and global symptoms in women with severe, diarrhea-predominant irritable bowel syndrome: analysis of two controlled trials. Clin Gastroenterol Hepatol. 2004;2:675–682. doi: 10.1016/s1542-3565(04)00284-8. [DOI] [PubMed] [Google Scholar]
- 102.Research Triangle Park, NC: GlaxoSmithKline; Lotronex[R] (alosetron hydrochloride) tablets [prescribing information] February 2005. [Google Scholar]
- 103.Chey WD, Chey WY, Heath AT, et al. Long-term efficacy and safety of alosetron in women with severe diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2004;99:2195–2203. doi: 10.1111/j.1572-0241.2004.30509.x. [DOI] [PubMed] [Google Scholar]
- 104.Chang L, Ameen VZ, Dukes GE, McSorley DJ, Carter EG, Mayer EA. A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS. Am J Gastroenterol. 2005;100:115–123. doi: 10.1111/j.1572-0241.2005.40365.x. [DOI] [PubMed] [Google Scholar]
- 105.Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT4 receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655–1666. doi: 10.1046/j.1365-2036.2001.01094.x. [DOI] [PubMed] [Google Scholar]
- 106.Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2002;16:1877–1888. doi: 10.1046/j.1365-2036.2002.01372.x. [DOI] [PubMed] [Google Scholar]
- 107.Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut. 2003;52:671–676. doi: 10.1136/gut.52.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nyhlin H, Bang C, Elsborg L, et al. A double-blind, placebo-controlled, randomized study to evaluate the efficacy, safety and tolerability of tegaserod in patients with irritable bowel syndrome. Scand J Gastroenterol. 2004;39:119–126. doi: 10.1080/00365520310006748. [DOI] [PubMed] [Google Scholar]
- 109.Bardhan KD, Forbes A, Marsden CL, Mason T, Short G. The effects of withdrawing tegaserod treatment in comparison with continuous treatment in irritable bowel syndrome patients with abdominal pain/discomfort, bloating and constipation: a clinical study. Aliment Pharmacol Ther. 2004;20:213–222. doi: 10.1111/j.1365-2036.2004.02032.x. [DOI] [PubMed] [Google Scholar]
- 110.Muller-Lissner S, Holtmann G, Rueegg P, Weidinger G, Loffler H. Tegaserod is effective in the initial and retreatment of irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2005;21:11–20. doi: 10.1111/j.1365-2036.2004.02294.x. [DOI] [PubMed] [Google Scholar]
- 111.East Hanover, NJ: Novartis Pharmaceuticals Corporation; Zelnorm [R] (tegaserod maleate) tablets [prescribing information] August 2004. [Google Scholar]
- 112.Tougas G, Snape WJ, Jr, Otten MH, et al. Long-term safety of tegaserod in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:1701–1708. doi: 10.1046/j.1365-2036.2002.01347.x. [DOI] [PubMed] [Google Scholar]
- 113.Coremans G, Clouse RE, Carter F, Krause G, Caras S, Steinborn C. Cilansetron, a novel 5-HT3 antagonist, demonstrated efficacy in males with irritable bowel syndrome with diarrhea-predominance (IBS-D) [abstract] Gastroenterology. 2004;126(suppl 2):A643. [Google Scholar]
- 114.Cilansetron: KC 9946. Drugs Res Dev. 2005;6:169–173. doi: 10.2165/00126839-200506030-00005. [DOI] [PubMed] [Google Scholar]
- 115.Bradette M, Moennikes H, Carter F, Krause G, Caras S, Steinborn C. Cilansetron in irritable bowel syndrome with diarrhea predominance (IBS-D): efficacy and safety in a 6 month global study [abstract] Gastroenterology. 2004;126:A42. [Google Scholar]
- 116.Clouse RE, Caras S, Cataldi F, Carter F, Krause G, Steinborn C. Cilansetron is efficacious for relief of urgency in patients with irritable bowel syndrome with diarrhea predominance (IBS-D). Program and abstracts of the First European Society for Pediatric Gastroenterology and Nutrition (ESPGHAN) Capri meeting; May 27-29, 2004; Naples, Italy. [Google Scholar]
- 117.Galligan JJ, Vanner S. Basic and clinical pharmacology of new motility promoting agents. Neurogastroenterol Motil. 2005;17:643–653. doi: 10.1111/j.1365-2982.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 118.Cremonini F, Talley NJ. Treatments targeting putative mechanisms in irritable bowel syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005;2:82–88. doi: 10.1038/ncpgasthep0096. [DOI] [PubMed] [Google Scholar]
- 119.Meyers NL, Palmer RMJ, George A. Efficacy and safety of renzapride in patients with constipation-predominant IBS: a phase IIb study in the UK primary healthcare setting [abstract] Gastroenterology. 2004;126:A640. [Google Scholar]
- 120.Henderson JC, Palmer RMJ, Meyers NL, Spiller RC. A phase IIb clinical study of renzapride in mixed-symptom (alternating) irritable bowel syndrome [abstract] Gastroenterology. 2004;126:A644. [Google Scholar]
- 121.Camilleri M, Kim D-Y, McKinzie S, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clinical gastroenterology and Hepatology. 2003;1:111–121. doi: 10.1053/cgh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 122.Delgado-Aros S, Chial HJ, Camilleri M, et al. Effects of a kappa-opioid agonist, asimadoline, on satiation and GI motor and sensory functions in humans. Am J Physiol Gastrointestinal Liver Physiol. 2003;284:G558–G566. doi: 10.1152/ajpgi.00360.2002. [DOI] [PubMed] [Google Scholar]
- 123.Camilleri M. Treating irritable bowel syndrome: overview, perspective and future therapies. Br J Pharmacol. 2004;141:1237–1248. doi: 10.1038/sj.bjp.0705741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Su X, Julia V, Gebhart GF. Effects of intracolonic opioid receptor agonists on polymodal pelvic nerve afferent fibers in the rat. J Neurophysiol. 2000;83:963–970. doi: 10.1152/jn.2000.83.2.963. [DOI] [PubMed] [Google Scholar]
- 125.Ozaki N, Sengupta JN, Gebhart GF. Differential effects of mu-, delta and kappa-opioid receptor agonists on the mechanosensitive gastric vagal afferent fibers in the rat. J Neurophysiol. 2000;83:2209–2216. doi: 10.1152/jn.2000.83.4.2209. [DOI] [PubMed] [Google Scholar]
- 126.Delvaux M, Beck A, Jacob J, et al. Effect of asimadoline, a kappa opioid agonist, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:237–246. doi: 10.1111/j.1365-2036.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 127.Lubiprostone: RU 0211, SPI 0211. Drugs Res Dev. 2005;6:245–248. doi: 10.2165/00126839-200506040-00009. [DOI] [PubMed] [Google Scholar]
- 128.Orr KK. Lubiprostone: a novel chloride channel activator for the treatment of constipation. Formulary. 2006;41:118–120. 122, 128–129. [Google Scholar]
- 129.Horvath EJ, Horvath K, Hamori T, et al. Anxiolytic 2,3, benzodiazepines, their specific binding to the basal ganglia. Prog Neurobiol. 2000;60:309–342. doi: 10.1016/s0301-0082(99)00020-9. [DOI] [PubMed] [Google Scholar]
- 130.Horvath EJ, Horvath K, Hamori T, Fekete MI, Solyom S, Palkovits M. Anxiolytic 2,3-benzodiazepines, their specific binding to the basal ganglia. Prog Neurobiol. 2000;60:309–342. doi: 10.1016/s0301-0082(99)00020-9. [DOI] [PubMed] [Google Scholar]
- 131.Leventer S, Raudibaugh K, Mangel A, et al. Dextofisopam improves bowel function in men and women with IBS. Am J Gastroenterol. 2005;100:S344. [Abstract #941] [Google Scholar]
- 132.Leventer S, Raudibaugh K, Frissora C, et al. The safety and efficacy of dextofisopam in patients with diarrhea-predominant or alternating irritable bowel syndrome. Gastroenterology. 2005;128:A94. doi: 10.1111/j.1365-2036.2007.03566.x. [DOI] [PubMed] [Google Scholar]
- 133.Pres release: Vela announces positive phase 2 results for dextofisopam in treating irritable bowel syndrome-IBS; results show effects of dextofisopam both in women and in men. Business Wire; January 6, 2005. Available at: http://www.centerwatch.com/bookstore/nmt/nmtb_ibs.pdf Accessed May 25, 2006.
- 134.Currie MG, Kurtz C, Mahajan-Miklos S, Busby RW, Fretzen A, Geis S. Effects of single dose administration of MD-1100 on safety, tolerability, exposure, and stool consistency in healthy subjects. Am J Gastroenterol. 2005;100:S328. [Abstract #894] [Google Scholar]
- 135.Press release: Microbia successfully completes phase IA trial of MD-1100 for IBS-C and other GI disorders. Microbia, Inc; May 17, 2005. Available at: http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/05-17-2005/0003633751&EDATE= Accessed May 25, 2006.
- 136.Phase 2 study of MD-1100 acetate on gastrointestinal transit in patients with C-IBS. Available at: http://www.clinicaltrials.gov/ct/show/NCT00258193?order=7 Accessed May 26, 2006.
- 137.Xing JH, Soffer EE. Adverse effects of laxatives. Dis Colon Rectum. 2001;44:1201–1209. doi: 10.1007/BF02234645. [DOI] [PubMed] [Google Scholar]
- 138.Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ. 1998;159:1245–1252. [PMC free article] [PubMed] [Google Scholar]
- 139.Camilleri M, Kim D-Y, McKinzie S, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2003;1:111–121. doi: 10.1053/cgh.2003.50019. [DOI] [PubMed] [Google Scholar]


