Figure 2.
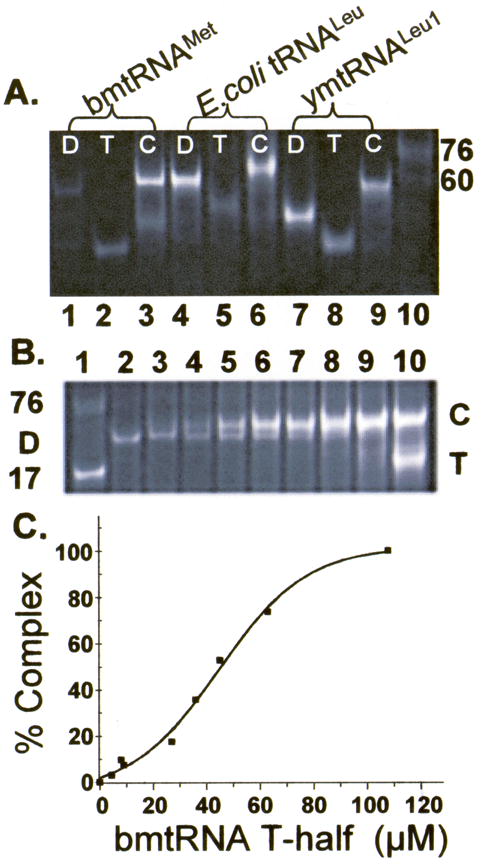
Gel mobility shift assay of tRNA folding interactions. A. The unmodified D- and T-domain half molecules of bmtRNAMet (lanes 1–3), E. coli tRNALeu-ΔACVL (E. coli tRNALeu, lanes 4–6) and ymtRNALeu1-ΔACVL (ymtRNALeu1, lanes 7–9) were subjected to polyacrylamide gel electrophoresis (4 °C) individually or together in the presence of 3 mM Mg2+. The individual half molecules, D (lanes 1, 4 and 7) and T (lanes 2, 5, and 8), and the D:T complex, C (lanes 3, 6, and 9), are denoted. The D- and T- half molecules of the three tRNAs migrate differently because they are of different lengths and nucleoside compositions. Two molecular size standards are also visible in the electrophoresis, a limited sample of unfractionated E. coli tRNA (3 μg) providing a broad band at ~76 nucleotides, and a 60mer RNA. B. Reconstitution of bmtRNAMet by formation of a complex between the unmodified D-domain half molecule titrated with the T-half molecule. The unmodified D-half molecule of bmtRNAMet (30 μM) was subjected to polyacrylamide gel electrophoresis (4 °C) in the presence of 3 mM Mg2+ and increasing concentrations of T-domain half molecule. Lane 1, standard 17 nucleotide ASL and unfractionated E. coli tRNA as markers; lane 2, 30 μM D-half molecule alone; lanes 3–9, 30 μM D-half molecule and increasing concentrations of the T-half molecule, lane 3, 4.8 μM, lane 4, 9.6 μM; lane 5, 19.3 μM; lane 6, 29 μM; lane 7, 39 μM; lane 8, 48.3 μM; lane 9, 68 μM; lane 10, 116 μM. Excess T-half molecule is evident in Lane 10. C. Binding of the unmodified bmtRNAMet D-half molecule to the unmodified T-half molecule in the presence of 3 mM Mg2+. Complex formed from titration of the D-half molecule with the T-half molecule and observed in PAGE (Fig. 2B) was quantified by analyzing the density of the bands. The percent of complex formed (relative to 100% in the presence of excess T-half molecule) is plotted as a function of T-half molecule concentration, generating a binding curve.
