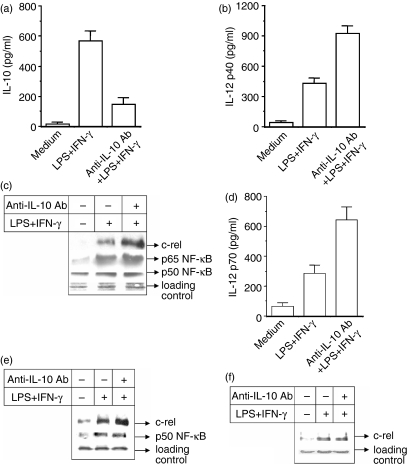
Figure 1.
Inhibition of endogenous IL-10 by treating macrophages with anti-IL-10 antibody (anti-IL-10 Ab) significantly increases nuclear c-rel level in addition to p65 NF-κB and causes up-regulation of IL-12 production. The RAW 264.7 macrophages were pretreated with 10 μg/ml anti-IL-10 Ab for 1 hr and further stimulated with LPS + IFN-γ for 48 hr. The production of IL-10 (a) and IL-12 p40 (b) was measured by EIA (mean ± SD). In another experiment, the macrophages were stimulated with LPS + IFN-γ for 1 hr in the absence or presence of anti-IL-10 Ab and the expression of c-rel and p65 and p50 NF-κB in the nuclear extracts was examined by immunoblot analysis of identical amounts of total proteins loaded in (c). IL-12 p70 level was measured by EIA in the IL-10-treated macrophages (d). In a separate experiment, the nuclear extracts prepared from RAW 264.7 macrophages activated with LPS + IFN-γ in the absence or presence of 10 μg/ml of anti-IL-10 Ab. The nuclear extracts were incubated first with anti-c-rel Ab overnight at 4° and then protein A/G–Sepharose was added to the mixture and it was incubated for a further 3 hr. Co-immunoprecipitated p50 was detected by Western blot analysis using the anti-p50 Ab(e, middle panel). The same preparation was also used to detect c-rel using anti-c-rel Ab, respectively (e; upper panel). In a complementary experiment, c-rel was coimmunoprecipitated using anti-p50 Ab and subjected to Western blotting for c-rel using anti-c-rel Ab (f). Results shown are representative of four independent experiments.