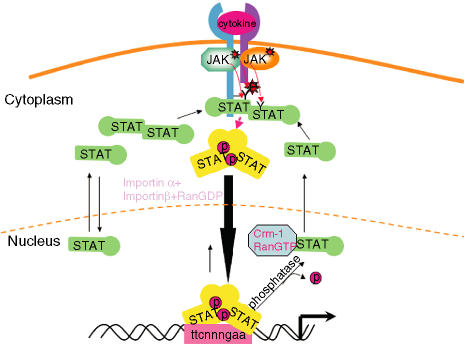
Figure 1.
The JAK-signal transducer and activator of transcription (JAK-STAT) signalling pathway. Ligand-induced activation of latent cytoplasmic JAK kinases leads to the docking and subsequent tyrosine phosphorylation of latent monomeric or N-domain-mediated dimeric STAT proteins, facilitating the formation of active SH2-domain-mediated homodimers of STAT proteins. The tyrosine-phosphorylated STAT dimers are actively transported to the nucleus using metabolic energy and the importin α/β and RanGDP complex. Once inside the nucleus, the active STAT dimers bind to the promoters of genes containing the consensus recognition motif (GAS motif-ttcnnngaa) and activate transcription of these genes. STATs can bind DNA as dimers or as N-domain-mediated tetramers. The active STAT protein is only released from DNA upon dephosphorylation by nuclear phosphatases, following cytokine withdrawal, and the inactivated protein is then actively exported out of the nucleus to the cytoplasm by the exportin Crm-1/RanGTP complex. Monomeric STAT proteins can also passively shuttle between the cytoplasm and the nucleus via carrier-free diffusion through nuclear pores facilitated by the interaction with nucleoporins.19