Abstract
The immune expulsion of gastrointestinal nematode parasites is usually associated with T helper type 2 (Th2) responses, but the effector mechanisms directly responsible for parasite loss have not been elucidated. The intestinal inflammatory response accompanying infection with gastrointestinal helminths is thought to be a contributory factor leading to the expulsion of the parasite. However, we have shown that the intestinal inflammation, which is controlled by interleukin (IL)-4, is not required for parasite expulsion. OX40–OX40 ligand (L) signals have been shown to be important for the development of Th2 immune responses but are also involved in a number of inflammatory diseases including those of the intestine. Here, we have investigated the effect of OX40 and OX40L fusion protein treatment on the induction of protective Th2 responses and enteropathy following infection with the gastrointestinal nematode Trichinella spiralis. Treatment with an OX40–immunoglobulin (Ig) blocking fusion protein resulted in enhanced expulsion of the parasite and an increase in the accompanying mastocytosis, despite unaltered levels of Th2 cytokines. Furthermore, there was a delay in the villus atrophy and crypt hyperplasia usually associated with this infection. In contrast, levels of Th2 cytokines were greatly up-regulated in mice treated with an OX40L–Ig activating fusion protein, yet the expulsion of the parasite and the enteropathy were unaffected. Therefore, OX40 ligation potentiates the Th2 response without enhancing host protective immune responses, whereas blocking the OX40–OX40L interaction enhances host protection without promoting Th2 cytokine responses during Trichinella spiralis infection.
Keywords: parasitic helminth, costimulation, mucosa, Th1/Th2, mast cells
Introduction
Gastrointestinal nematodes cause some of the most prevalent and chronic human diseases in the world.1 Protective immunity to most gut helminths, including Trichinella spiralis, is mediated by CD4+ T helper (Th) cells generated in the mesenteric lymph nodes.2,3 Various studies have indicated a requirement for interleukin (IL)-4 and IL-13 in the expulsion of gastrointestinal helminths, with parasite loss being delayed or prevented in IL-4–/– mice, interleukin-4 receptor α-chain (IL-4Rα)–/– mice and signal transducers and activators of transcription (STAT)-6–/– mice.4–6 Immune expulsion of adult T. spiralis worms parasitizing the small intestine is a complex process associated with a Th2-mediated eosinophilia, goblet cell hyperplasia and mastocytosis; however, the effector mechanisms have yet to be elucidated.5,6 Although intestinal inflammation accompanies worm expulsion in mice, there is mounting evidence demonstrating that enteropathy is not a requirement for parasite loss.7 For example, both tumour necrosis factor receptor 1 (TNFR1)–/– and inducible nitric oxide synthase (iNOS)–/– mice have reduced villus atrophy and crypt cell hyperplasia but are still able to expel T. spiralis.8 In contrast, IL-4–/– mice have reduced pathology with delayed parasite expulsion, indicating that TNF-α-mediated enteropathy may be controlled by IL-4.7
During an immune response, effective T-cell activation requires two signals. Following interaction of the T-cell receptor (TCR) with antigen on major histocompatibility complex (MHC) molecules, a second signal is provided by costimulatory molecules. These include several TNF receptor-related proteins such as CD40, CD30, 4-1BB and OX40,9,10 with their corresponding type II transmembrane protein ligands CD40L, CD30L, 4-1BBL and OX40L. 11–13 Costimulation of T cells via these receptor–ligand interactions is required for full activation in response to antigen and, in the absence of costimulation, recognition of antigen can lead to tolerance induction.14 OX40 is expressed solely by activated T cells, being up-regulated 48 hr after antigen stimulation and disappearing after 72–96 hr.14,15 However, OX40L has a wide tissue distribution and is expressed predominantly by dendritic cells,16 B cells17 and endothelial cells under inflammatory conditions.18 It has been shown that costimulation via the OX40 receptor can bias the immune response towards a Th2 phenotype,19 but the interaction has also been implicated in a number of inflammatory diseases including Th1-mediated intestinal inflammation.20 Blocking OX40L using either an OX40–immunoglobulin (Ig) fusion protein or a neutralizing anti-OX40L antibody suppressed ongoing colitis,20 reduced T-cell immunopathology during influenza virus infection without compromising viral clearance,21 and ameliorated experimental allergic encephalomyelitis (EAE) by preventing migration of pathogenic T cells to the central nervous system.18,22 In addition, blocking OX40L, using a neutralizing anti OX40L antibody, inhibited disease progression in Leishmania major infection in susceptible mice23 by reducing the Th2 response. However, ligating OX40 with OX40L–Ig has been shown to enhance protective responses against the Th1-inducing lung pathogen Cryptococcus neoformans.24
In this study, the effects of OX40–Ig blocking and OX40L–Ig activating fusion proteins on the regulation of protective Th2 responses and enteropathy following infection with the gastrointestinal nematode T. spiralis were examined. We show here that blockade of OX40L, using an OX40–Ig fusion protein, enhanced expulsion of T. spiralis from the gut and that this was associated with a prominent mucosal mastocytosis. During the early stages of infection, villus atrophy and crypt hyperplasia were significantly reduced following OX40–Ig treatment. Paradoxically, these effects occurred without alteration of the T. spiralis-associated Th2 cytokine response in OX40–Ig fusion protein-treated mice. Conversely, in vivo engagement of T cells by OX40L–Ig fusion protein potentiated the Th2 response in the context of T. spiralis infection, but this did not enhance expulsion of the parasite or promote mucosal mastocytosis and enteropathy.
Materials and methods
Mice
Female 6–8-week-old BALB/c mice were purchased from Harlan-Olac (Bicester, UK) and maintained in the University of Strathclyde animal facility. All mice were housed under standard conditions with free access to food and water. Procedures were performed under Home Office regulations.
Infection of mice
T. spiralis larvae were maintained by serial passage in CD1 mice and recovered from infected mice as described previously.25 BALB/c mice were infected orally with 400 T. spiralis larvae and killed at various times post infection.
Treatment with OX40 and OX40L fusion proteins
Mouse (m) OX40–mIgG1 and mOX40L–mIgG1 fusion proteins were constructed as described previously.26,27T. spiralis-infected and uninfected controls were injected intraperitoneally (i.p.) with mOX40–Ig (100 µg) or mOX40L–Ig (100 µg) on days 2, 5 and 8 post infection, as OX40 is only expressed 2–3 days following T-cell activation, with expression decreasing by 4–5 days following T-cell activation.14,15 Groups of five mice were killed on days 7 and 14 post infection and assessments on protection and pathology made.
Recovery of adult worms from the small intestine
The small intestine was opened longitudinally, wrapped in mesh squares and incubated in Hanks balanced salt solution (HBSS) at 37° for 3 hr, to induce migration of worms from the gut epithelium into solution. Following incubation, the mesh squares containing the guts were agitated to release any trapped worms. The worms were counted using a scored Petri dish and an inverted dissecting microscope.
Assessment of cytokine production
Mesenteric lymph nodes were removed under sterile conditions and single-cell suspensions were prepared by forcing the tissue through sterile Nitex membranes (Cadischprecision Meshes, London, UK) in RPMI 1640 (Gibco, Paisley, UK) supplemented with 25 mm HEPES, 10% fetal calf serum, 5 mm l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, amphotericin B and 0·05 mβ-mercaptoethanol (all Gibco). Cell aggregates were removed by passing cells through sterile Nitex membranes. Viable cells were counted using the trypan blue exclusion assay and 1 × 106 cells/100 µl were incubated in sterile 96-well microtitre plates with or without 50 µg/ml T. spiralis antigen. Briefly, T. spiralis antigen was prepared by homogenization of T. spiralis larvae followed by several rounds of centrifugation at 9000 gfor 5 min and re-homogenization of the pellet in sterile phosphate-buffered saline (PBS). Following a 24-hr incubation at 37° with 5% CO2, the cells were centrifuged at 400 g. The cell supernatants were collected and frozen in fresh 96-well plates at −20° for cytokine assays. Cytokines present in tissue culture supernatants were determined using sandwich enzyme-linked immunosorbent assays (ELISAs) for IL-4 (Biosource International, Camarillo, CA), IL-13 (R&D, Abingdon, UK), IL-18 (BD PharMingen, San Diego, CA) and interferon (IFN)-γ (Biosource International).
Measurement of antibody responses
Parasite-specific IgG1 and IgG2a and total IgE levels were determined as described previously with minor modifications.7 Briefly, T. spiralis larval homogenate was used as a target antigen at 2 µg/ml. Sera were doubly diluted starting at 1/40. IgG1 and IgG2a isotypes were detected using horseradish peroxidase (HRP) conjugated anti-mouse IgG1 and IgG2a at 1/10 000 dilution (Southern Biotech, Cambridge, UK). Total IgE levels were measured using a sandwich ELISA as described previously.7 Absorbances were measured at 405 nm, reference 650 nm, using a Spectramax ELISA reader (Molecular Devices, Wokingham, UK).
Intestinal pathology assessment
Samples (1 cm) of jejunum were taken 10 cm from the pylorus, opened longitudinally, and then fixed in Clarke's fixative (25% acetic acid/75% ethanol). After 24 hr, the fixative was replaced with 75% ethanol and the gut sections permeabilized using 1 m HCl at 60° for 7 min followed by staining with Schiff reagent (Sigma, Poole, UK). Sections were microdissected as described previously7 and villus and crypt lengths measured using an eyepiece micrometer. Ten villi and crypt units were measured for each sample and the mean villus and crypt length determined for each. The mean number of mitotic figures in 10 randomly selected crypt areas was also determined.
Mast cell quantification
Jejunum samples 2–3 cm long taken 10 cm from the pylorus were rolled, villi outermost, using the Swiss roll technique and then fixed for 1 hr in Carnoy's fixative followed by processing using standard histological techniques. Sections were stained with 0·5% toluidine blue (Sigma) in 0·5 M HCl for mast cell visualization7 and counterstained with 0·5% Safranine O (Sigma) for 2 min. The number of mucosal mast cells (MMCs) was counted in 10 villus/crypt units (VCU) and data expressed as mean number of MMCs per VCU.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Significant differences between means were determined using analysis of variance (ANOVA) followed by Fisher's least significant difference post-hoc analysis. A P-value of < 0·05 was considered significant.
Results
OX40 fusion protein enhanced T. spiralis expulsion from the small intestine
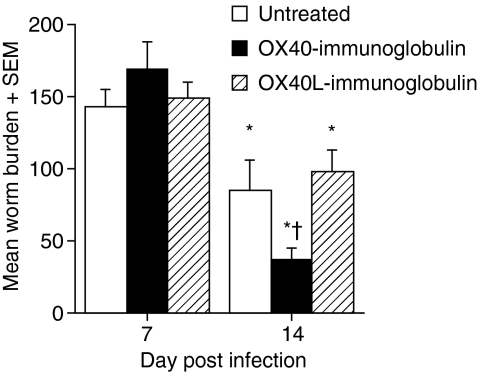
OX40–Ig and OX40L–Ig were used to block and activate OX40, respectively. Blockade of OX40 interactions with OX40–Ig inhibits mitogen and antigen-specific proliferation in vitro in a dose-dependent manner and T-cell proliferation and extravasation in vivo.20,21,26 Conversely, engagement of OX40 receptors with OX40L–Ig delivers a potent costimulatory signal to effector T cells both in vitro and in vivo.24,27,28 The effects of OX40–Ig and OX40L–Ig fusion proteins on expulsion of adult T. spiralis worms were investigated following oral inoculation with 400 T. spiralis larvae. Previous studies have shown that expulsion of T. spiralis is first evident at 12 days post infection (d.p.i.) with significant loss occurring by 14 d.p.i.7 In the present study, there were no differences in the establishment of the worm population at 7 d.p.i. following treatment with either fusion protein. Figure 1 reveals that OX40–Ig fusion protein-treated mice had significantly reduced worm burdens as compared with untreated mice by 14 d.p.i. (P < 0·05) while expulsion of parasites from OX40L–Ig-treated mice was similar to that from untreated mice.
Figure 1.
In vivo treatment with OX40–immunoglobulin (Ig) significantly enhanced worm expulsion from the gut. Trichinella spiralis-infected mice were treated with either OX40 or OX40 ligand (OX40L)–Ig fusion protein. Mice were killed at 7 and 14 days post infection and adult worms within the small intestine were counted. *, Significantly different from uninfected mice; †, significantly different from infected untreated mice (P < 0·05). Data are presented as the mean gut burden + the standard error of the mean for five mice per group. Results shown are representative of two independent experiments.
Effect of OX40 and OX40L fusion protein treatment on cytokine production during T. spiralis infection
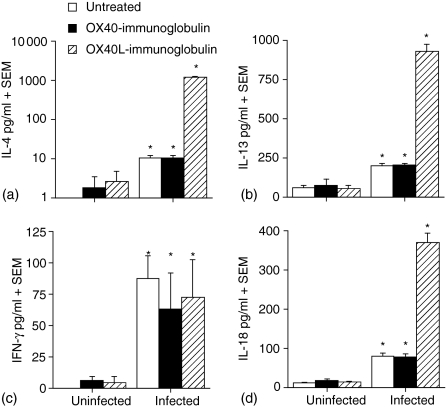
The Th2 cytokines IL-4 and IL-13 are central in protection of the host against T. spiralis infection. The cytokine responses in fusion protein-treated, T. spiralis-infected mice were determined following restimulation of mesenteric lymph node (MLN) cells in vitro with T. spiralis antigen. Figures 2(a) and 2(b) demonstrate that both OX40–Ig-treated and untreated mice had an increase in the production of the Th2 cytokines IL-4 and IL-13 following infection with T. spiralis. Treatment of mice with OX40L–Ig fusion protein resulted in the development of a very potent Th2 response as compared with both OX40–Ig-treated and untreated mice, whereby IL-4 (Fig. 2a) and IL-13 (Fig. 2b) cytokines were increased approximately 100-fold (P < 0·001). Levels of IL-3, IL-5 and IL-9 were also examined and displayed a similar pattern of response to IL-4 and IL-13 (data not shown). As has been shown previously,7 levels of IFN-γ were not altered following infection with T. spiralis and neither OX40–Ig nor OX40L–Ig fusion protein up-regulated the IFN-γ response (Fig. 2c). The proinflammatory cytokine IL-18 has been shown to regulate expulsion of gastrointestinal helminths; treatment of T. spiralis-infected mice with recombinant IL-18 results in a delayed loss of parasites.29 Our data showed that untreated and OX40–Ig-treated animals produced similar levels of IL-18 following infection; however, OX40L–Ig treatment significantly up-regulated IL-18 production (P < 0·05) in a similar manner to IL-4 and IL-13, but this had no apparent effect on the kinetics of T. spiralis expulsion (Fig. 2d).
Figure 2.
Up-regulation of T helper type 2 (Th2)-associated cytokines in the mesenteric lymph node (MLN) during Trichinella spiralis infection following in vivo treatment with OX40 ligand (OX40L)–immunoglobulin (Ig) fusion protein. MLNs were removed, single cell suspensions prepared and MLN cells stimulated with 50 µg/ml T. spiralis antigen. Supernatants were analysed by sandwich enzyme-linked immunosorbent assay (ELISA) for the presence of interleukin (IL)-4, (b) IL-13, (c) interferon (IFN)-γ and (d) IL-18. *, Significantly different from uninfected mice; †, significantly different from infected untreated mice (P < 0·05). Data are presented as the mean cytokine concentration (pg/ml) + the standard error of the mean for five mice per group. Results shown are representative of two independent experiments.
Treatment with OX40L fusion protein enhanced total IgE but not IgG1 or IgG2a levels during T. spiralis infection
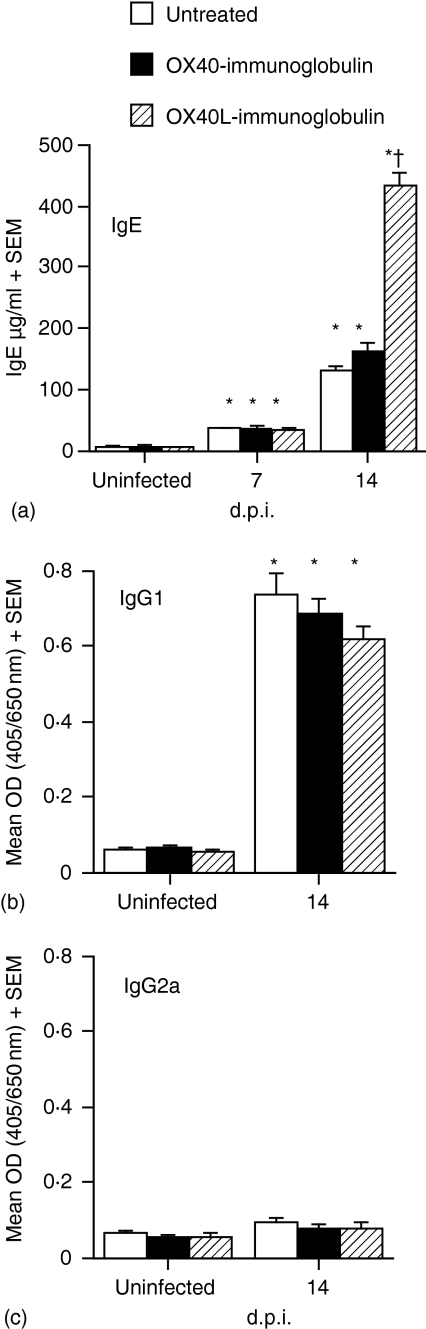
As Th2 cytokines were significantly up-regulated in OX40L–Ig-treated mice, the effects on the subsequent immunoglobulin response were investigated. Consistent with previous observations,7 infection with T. spiralis resulted in elevated levels of IgE. While treatment with OX40–Ig did not alter the IgE response significantly, total serum IgE levels were significantly enhanced in OX40L–Ig-treated mice (P < 0·001) as compared with both OX40–Ig-treated and untreated mice (Fig. 3a). Treatment with either OX40–Ig or OX40L–Ig did not result in any significant differences in IgG1 levels, with significantly elevated levels observed following parasite infection in all cases (Fig. 3b). IgG2a responses were not significantly altered following infection or treatment with either fusion protein (Fig. 3c).
Figure 3.
In vivo treatment with OX40 ligand (OX40L)–immunoglobulin (Ig) fusion protein enhanced IgE synthesis in T. spiralis-infected mice but did not affect IgG1 or IgG2a production. Total serum IgE (a) and T. spiralis antigen-specific IgG1 (b) and IgG2a (c) concentrations were determined by enzyme-linked immunosorbent assay (ELISA). IgE concentrations are expressed as the mean + the standard error of the mean for five mice per group. T. spiralis antigen-specific IgG1/IgG2a levels are expressed as mean optical density (OD) + the standard error of the mean for five mice per group. *, Significantly different from uninfected mice; †, significantly different from infected untreated mice (P < 0·001). Results shown are representative of two independent experiments.
Treatment with OX40 fusion protein but not OX40L fusion protein delayed villus atrophy during early stages of T. spiralis infection
It has been previously shown that T. spiralis-associated enteropathy is not a prerequisite for expulsion of adult parasites.7 As OX40–Ig fusion protein had been shown to ameliorate ongoing colitis in mice,20 the effects of fusion protein treatment on T. spiralis-associated enteropathy were investigated. Changes in villus and crypt length and crypt mitotic activity in microdissected samples of jejunum were assessed.
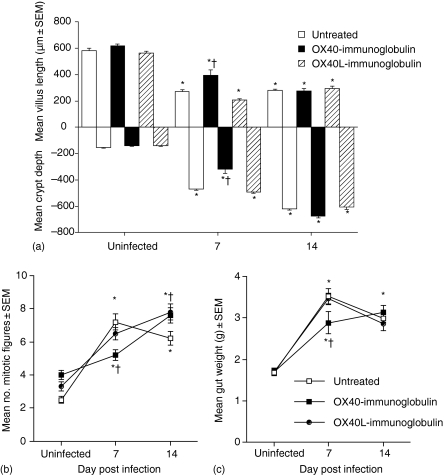
Uninfected mice showed no evidence of intestinal abnormalities, regardless of fusion protein treatment (Fig. 4a). As expected, by 7 d.p.i., untreated mice demonstrated severe gut pathology characterized by significant villus atrophy, crypt hyperplasia and an increase in the number of mitotic figures (Figs 4a and b). A similar degree of pathology was observed in OX40L–Ig fusion protein-treated mice at 7 d.p.i., with no significant differences observed. In contrast, T. spiralis-infected mice treated with OX40–Ig fusion protein displayed a significant reduction in villus atrophy, crypt hyperplasia and numbers of mitotic figures at 7 d.p.i. (P < 0·05) compared with untreated and OX40L–Ig-treated groups (Figs 4a and b). However, the reduction in enteropathy observed in OX40–Ig-treated mice was not sustained, as villus atrophy (Fig. 4a), crypt hyperplasia (Fig. 4a) and number of mitotic figures (Fig. 4b) were not significantly different from either untreated or OX40L–Ig-treated animals at 14 d.p.i.
Figure 4.
In vivo treatment with OX40–immunoglobulin (Ig) significantly delayed intestinal pathology during early infection with T. spiralis. The lengths of villi and crypts (a) were measured and the mean number of mitotic figures per crypt (b) determined at 7 and 14 days post infection (d.p.i.). The entire small intestine from infected and uninfected mice was weighed at 7 and 14 d.p.i. (c). *, significantly different from uninfected mice; †, significantly different from infected untreated mice (P < 0·05). Data are presented as mean villus/crypt length + standard error of the mean (SEM), mean number of mitotic figures + SEM and mean wet weight (g) + SEM, respectively. Five mice were used per group and the results shown are representative of two independent experiments.
Gut weight was also measured as an indicator of intestinal inflammation (Fig. 4c). At 7 d.p.i., the weight of guts from untreated and OX40L–Ig-treated mice had increased significantly; however, those of OX40–Ig-treated mice were significantly lower than those of either untreated or OX40L–Ig-treated mice. By 14 d.p.i., gut weights were significantly greater than those of uninfected animals, with no significant differences observed between any of the infected groups.
Treatment with OX40 fusion protein enhanced mucosal mastocytosis during T. spiralis infection
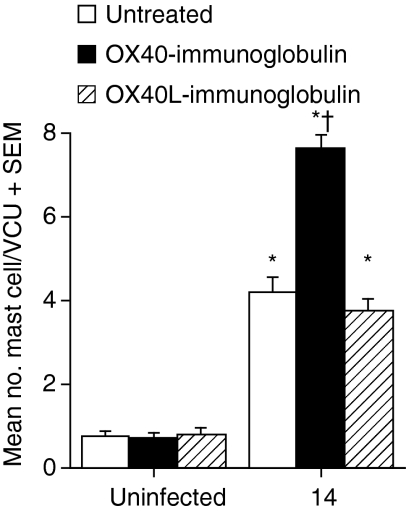
Mast cells are potent effector cells involved in expulsion of T. spiralis from the gut30,31 and are regulated by Th2 cytokines as well as IL-18. To investigate whether the increased expulsion of parasites from the small intestine of OX40–Ig-treated mice was associated with an enhanced mastocytosis, we enumerated mucosal mast cells in the small intestine. While infection with T. spiralis resulted in a similar mastocytosis in both untreated and OX40L–Ig-treated animals, treatment with OX40–Ig resulted in significantly elevated MMC numbers (P < 0·001) compared with both untreated and OX40L–Ig-treated mice (Fig. 5). Analysis of levels of murine mast cell protease 1 (mMCP-1) showed a similar pattern, with increased levels in both untreated and OX40L–Ig-treated mice and significantly higher levels in OX40–Ig-treated mice (data not shown). Treatment with either fusion protein had no effect on either MMC numbers or levels of mMCP-1 in uninfected mice. The distribution of mast cells within the lamina propria and epithelium was similar for treated and untreated mice (data not shown).
Figure 5.
In vivo treatment with OX40–immunoglobulin (Ig) fusion protein, but not OX40 ligand (OX40L)–Ig, enhanced mucosal mastocytosis during T. spiralis infection. Carnoy's fixed gut tissue from uninfected and infected mice at 14 days post infection was stained with 0·5% toluidine blue to reveal mucosal mast cells (MMCs). MMCs were counted in at least 10 randomly selected villus crypt units (VCU). Data are expressed as mean number of MMCs per VCU + standard error of the mean for five mice per group. *, Significantly different from uninfected mice; †, significantly different from infected untreated mice (P < 0·05). Results shown are representative of two independent experiments.
Discussion
OX40–OX40L signalling has been shown to be important for the development of Th2 immune responses but are also involved in a number of inflammatory diseases, including those of the intestine. We have shown that in vivo treatment with a blocking OX40–Ig fusion protein enhanced expulsion of adult T. spiralis from the small intestine, and this was associated with an enhanced mucosal mastocytosis. Despite a reduction in intestinal pathology there was no apparent effect on Th2 cytokine production. In contrast, activation with OX40L–Ig fusion protein dramatically enhanced Th2 cytokine production, but this had no effect on mastocytosis, intestinal pathology or parasite expulsion.
Previous studies showed that IL-4 and IgE production induced during both primary and memory immune responses to Heligmosomoides polygyrus infection were partially inhibited in OX40–/– mice, although IgG1, germinal centre formation and antigen-specific Th2 expansion and migration to the B-cell area were unaffected. In contrast, protective immunity and the associated primary Th2 response were not inhibited in OX40L–/– mice infected with the gastrointestinal helminth Nippostrongylus brasiliensis.32 Similarly, our results demonstrate that blockade of OX40 had no effect on Th2 cytokine production or IgG1 or IgE antibody levels. The differences observed between these studies may be attributable to the differential requirement for signalling via other costimulatory molecules such as CD28 and/or inducible costimulator (ICOS) upstream of OX40–OX40L interactions, as blockade of these molecules appears to have a greater effect on protective immunity in a number of gastrointestinal helminth infections.33–37 Indeed, we have recently shown that blockade of ICOS can reduce Th2 responses and the intestinal inflammation accompanying infection with T. spiralis.38
In contrast to OX40–Ig treatment, ligation of OX40 with OX40L–Ig potentiated the Th2 cytokine response by markedly enhancing IL-4 and IL-13 production. OX40 only appears to have a marginal effect on either Th1 or Th2 commitment, but as IL-4 abrogates OX40L expression more efficiently than Th1 cytokines, thus removing the check on T cell proliferation, Th2 cells can out-proliferate their Th1 counterparts. Furthermore, as OX40L is still expressed on Th0 cells, OX40 signals can significantly influence the number of Th2 cells generated in an immune response.39 This has been suggested as a mechanism by which blockade of OX40 increases Th1 responses and resistance to disease during infection with Leishmania.23,32,40 Furthermore, this may explain how OX40L–Ig treatment markedly increased the Th2 responses in T. spiralis infection where significant levels of IL-4 were produced early in the course of infection, and also why lack of OX40 blockade had little effect on Th2 levels. Expulsion of T. spiralis is dependent on IL-4, IL-4Rα and STAT-6 signalling4–6 and, although the increased levels of Th2 cytokines did not lead to enhanced parasite loss, it is possible that, once a threshold level of Th2 cytokines, including IL-4, has been reached, worm expulsion may occur. Furthermore, the kinetics of the Th2 response may be important in determining the rate of expulsion and, as OX40 costimulation occurs 2–3 days following T-cell activation, OX40L–Ig treatment may serve to amplify the T-cell response induced rather than altering the time–course of the response.14,15 The enhancement of Th2 responses supports the findings of previous studies that have demonstrated the requirement for OX40 stimulation in IL-4 production.19,40 For example, OX40L transgenic mice which have constitutive costimulation of OX40 generate elevated Th2 responses characterized by increased IL-4, IL-5, IL-10 and IL-13 levels and unaltered IFN-γ responses following immunization of mice,41 while the absence of OX40 during allergic inflammation results in a striking reduction in eosinophil infiltration and production of Th2 cytokines.42 In agreement with previous studies,43 IgG1 and IgG2a levels were not affected by OX40 and/or OX40L modulation. Interestingly, IL-18 levels produced were also highly up-regulated following OX40L–Ig treatment, and this may account for the increase in Th2 cytokines and IgE, as it was previously shown that these responses were increased by IL-18 in a mouse model of allergic sensitization.44
Mast cells are key effector cells in mediating T. spiralis expulsion from the small intestine,31,45–47 and the increase in parasite loss may therefore be explained by the correlation with the enhanced mastocytosis observed following OX40–Ig treatment. The reasons for the increased mastocytosis following OX40 blockade are unclear at present, but this may be a result of increased mast cell extravasation and/or proliferation as, in addition to its costimulatory role, OX40 appears to contribute to T-cell migration and tissue infiltration through its interaction with OX40L on endothelial cells.48 Furthermore, alterations in integrin and/or chemokine and cytokine expression in intestinal tissue may also influence the development of a mastocytosis as these may be regulated in part by OX40 interactions.49,50 Importantly, OX40L–Ig treatment did not appear to potentiate the mast cell response. The elevated levels of IL-18 in OX40L–Ig-treated mice may account for the lack of an enhanced mastocytosis, as treatment of mice infected with T. spiralis in vivo with recombinant IL-18 resulted in suppressed mastocytosis and subsequent decreased host protection.29 Mast cells have been found to activate T cells and to induce T-cell proliferation by OX40 ligand,49 and we have recently shown that mast cells are an important source of Th2 cytokines, including IL-4.45 Studies of helminth infections in OX40- or OX40L-deficient mice have not reported any effects on the development of mastocytosis; however, the parasites used in these cases (N. brasiliensis and H. polygyrus) do not generate a profound mast cell response and mast cells are not required for parasite loss.32,35 More recent evidence suggests that triggering OX40 blocks the inhibitory activity of T cells51 and as T regulatory (Treg) cells can directly or indirectly influence a number of effector cells, including mast cells, blockade of OX40 could lead to unregulated proliferation of mast cells.52 Thus the role of OX40 interactions in the development of a mastocytosis warrants further study.
Previous studies using Th1 experimental models of inflammation have shown that OX40–Ig can reduce various inflammatory parameters and even ameliorate clinical symptoms. Prevention of CD4+ T-cell infiltration into the lamina propria of colonic villi during trinitrobenzesulphonic acid (TNBS) -induced or IL-2–/– models of colitis was achieved using in vivo OX40–Ig fusion protein treatment.20 Similarly, if OX40 fusion protein was administered at the onset of EAE, a reduction in clinical signs of the disease was observed.22 Furthermore, treatment with anti-OX40L monoclonal antibody completely blocked development of colitis, and it was suggested that this was via inhibition of T-cell proliferation and expression of α4β7.53 In our model, we propose that OX40–Ig may be acting to reduce T. spiralis-associated enteropathy in the small intestine by blocking the interaction of activated OX40+ T cells with OX40L+ vascular endothelial cells,53 thus preventing the extravasation of T cells into the intestinal mucosa. A single treatment of OX40–Ig was sufficient to ameliorate ongoing colitis in a mouse model.20 Although blockade of OX40 resulted in a significant amelioration of the enteropathy induced by T. spiralis infection, this was not sustained. The difference in the effectiveness of OX40–Ig treatment may be a reflection of the aetiologies of the disease, with colitis being a Th1-mediated enteropathy54 while that induced by T. spiralis is mediated by Th2 cytokines.7
Overall, our data provide evidence for the first time that the in vivo blocking of OX40L using OX40–Ig enhances the rapid expulsion of T. spiralis. As other studies have demonstrated that the Th2 response is attenuated in OX40L-deficient mice during allergic inflammation,35,55 it is surprising that the T. spiralis-specific Th2 response was unaltered following OX40–Ig treatment. However, alternative costimulatory pathways such as the ICOS/B7 homologous protein interaction33 may compensate in the absence of a functional OX40–OX40L pathway. The correlation of an enhanced mastocytosis and enhanced parasite loss at the same stage of infection suggests that OX40–Ig is having more subtle effects on the Th2-associated effector responses, namely the modulation of mast cell infiltration into the intestinal mucosa. In contrast, we showed that in vivo ligation of OX40 using OX40L–Ig potentiated the Th2 response, but this had no effect on protective responses against T. spiralis, suggesting that an increase in the Th2 environment beyond a threshold level has no beneficial effects on parasite loss or pathology.
Acknowledgments
This work was supported by a Wellcome Trust Grant (No 62264) to CEL.
Abbreviations
- d.p.i.
days post infection
- lg
immunoglobulin
- IL
interleukin
- L
ligand
- m
mouse
- mMCP-1
mouse mast cell protease 1
- MLN
mesenteric lymph node
- MMC
mucosal mast cells
- TH
T helper
- TNF
tumour necrosis factor
References
- 1.Chan MS, Medley GF, Jamison D, Bundy DA. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology. 1994;109:373–87. doi: 10.1017/s0031182000078410. [DOI] [PubMed] [Google Scholar]
- 2.Manson-Smith DF, Bruce RG, Parrott DM. Villous atrophy and expulsion of intestinal Trichinella spiralis are mediated by T cells. Cell Immunol. 1979;47:285–92. doi: 10.1016/0008-8749(79)90338-1. [DOI] [PubMed] [Google Scholar]
- 3.Garside P, Grencis RK, Mowat AM. T lymphocyte dependent enteropathy in murine Trichinella spiralis infection. Parasite Immunol. 1992;14:217–25. doi: 10.1111/j.1365-3024.1992.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 4.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–55. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence CE. Is there a common mechanism of gastrointestinal nematode expulsion? Parasite Immunol. 2003;25:271–81. doi: 10.1046/j.1365-3024.2003.00630.x. [DOI] [PubMed] [Google Scholar]
- 6.Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998;28:1145–58. doi: 10.1016/s0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence CE, Paterson JC, Higgins LM, MacDonald TT, Kennedy MW, Garside P. IL-4-regulated enteropathy in an intestinal nematode infection. Eur J Immunol. 1998;28:2672–84. doi: 10.1002/(SICI)1521-4141(199809)28:09<2672::AID-IMMU2672>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence CE, Paterson JC, Wei XQ, Liew FY, Garside P, Kennedy MW. Nitric oxide mediates intestinal pathology but not immune expulsion during Trichinella spiralis infection in mice. J Immunol. 2000;164:4229–34. doi: 10.4049/jimmunol.164.8.4229. [DOI] [PubMed] [Google Scholar]
- 9.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–62. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 10.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes – a molecule related to nerve growth factor receptor. EMBO J. 1990;9:1063–8. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1996;85:3378–404. [PubMed] [Google Scholar]
- 12.Baum PR, Gayle RB, Ramsdell F, et al. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godfrey WR, Fagnoni FF, Harara MA, Buck D, Engelman EG. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med. 1994;180:757–62. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal-Pakala P, Jember A, Croft M. Signalling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–12. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 15.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2001;165:3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 16.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–48. [PubMed] [Google Scholar]
- 17.Stuber E, Neurath MF, Calderhead D, Fell HP, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–21. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 18.Nohara C, Akiba H, Nakajima A, et al. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development of pathogenic T cells. J Immunol. 2001;166:2108–15. doi: 10.4049/jimmunol.166.3.2108. [DOI] [PubMed] [Google Scholar]
- 19.Ohshima H, Yang LP, Uchiyama T, Tanaka T, Baum P, Sergerie M, Hermann P, Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 20.Higgins LM, McDonald SA, Whittle N, Crockett N, Shields JG, MacDonald TT. Regulation of T cell activation in vitro and in vivo by targeting the OX40–OX40 ligand interaction: amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J Immunol. 1999;162:486–93. [PubMed] [Google Scholar]
- 21.Humphreys IR, Walzl G, Edwards L, Rae A, Hill S, Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med. 2003;198:1237–42. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162:1818–26. [PubMed] [Google Scholar]
- 23.Akiba H, Miyahira Y, Atsuta M, et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med. 2000;191:375–80. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphreys IR, Edwards L, Walzl G, Rae AJ, Dougan G, Hill S, Hussell T. OX40 ligation on activated T cells enhances the control of Cryptococcus neoformans and reduces pulmonary eosinophilia. J Immunol. 2003;170:6125–32. doi: 10.4049/jimmunol.170.12.6125. [DOI] [PubMed] [Google Scholar]
- 25.Wakelin D, Wilson MM. Transfer of immunity to Trichinella spiralis in the mouse with mesenteric lymph node cells: time of appearance of effective cells in donors and expression of immunity in recipients. Parasitology. 1977;74:215–24. doi: 10.1017/s0031182000047843. [DOI] [PubMed] [Google Scholar]
- 26.Taylor L, Bachler M, Duncan I, Keen S, Fallon R, Mair C, MacDonald TT, Schwarz H. In vitro and in vivo activities of OX40 (CD134)–IgG fusion protein isoforms with different levels of immune-effector functions. J Leukoc Biol. 2002;72:522–9. [PubMed] [Google Scholar]
- 27.Morris A, Vetto JT, Ramstad T, Funatake CJ, Choolu E, Entwistle C, Weinberg AD. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res Treat. 2001;67:71–80. doi: 10.1023/a:1010649303056. [DOI] [PubMed] [Google Scholar]
- 28.Zubairi S, Sanos SL, Hill S, Kaye PM. Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing of Leishmania donovani. Eur J Immunol. 2004;34:1433–40. doi: 10.1002/eji.200324021. [DOI] [PubMed] [Google Scholar]
- 29.Helmby H, Grencis RK. IL-18 regulates intestinal mastocytosis and Th2 cytokine production independently of IFN-gamma during Trichinella spiralis infection. J Immunol. 2002;169:2553–60. doi: 10.4049/jimmunol.169.5.2553. [DOI] [PubMed] [Google Scholar]
- 30.Vallance BA, Blennerhassett PA, Huizinga JD, Collins SM. Mast cell-independent impairment of host defense and muscle contraction in T. spiralis-infected W/W (v) mice. Am J Physiol Gastrointest Liver Physiol. 2001;280:G640–8. doi: 10.1152/ajpgi.2001.280.4.G640. [DOI] [PubMed] [Google Scholar]
- 31.Grencis RK, Else KJ, Huntley JF, Nishikawa SI. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 1993;15:55–9. doi: 10.1111/j.1365-3024.1993.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 32.Pippig SD, Pena-Rossi C, Long J, et al. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40) J Immunol. 1999;163:6520–9. [PubMed] [Google Scholar]
- 33.Coyle AJ, Lehar S, Lloyd C, et al. The CD28-related molecule ICOS is required for effective T cell- dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 34.Urban J, Fang H, Liu Q, et al. IL-13-mediated worm expulsion is B7 independent and IFN-gamma sensitive. J Immunol. 2000;164:4250–6. doi: 10.4049/jimmunol.164.8.4250. [DOI] [PubMed] [Google Scholar]
- 35.Ekkens MJ, Liu Z, Liu Q, et al. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J Immunol. 2003;170:384–93. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 36.Ekkens MJ, Liu Z, Liu Q, et al. Memory Th2 effector cells can develop in the absence of B7-1/B7-2, CD28 interactions, and effector Th cells after priming with an intestinal nematode parasite. J Immunol. 2002;168:6344–51. doi: 10.4049/jimmunol.168.12.6344. [DOI] [PubMed] [Google Scholar]
- 37.Greenwald RJ, Lu P, Halvorson MJ, et al. Effects of blocking B7-1 and B7-2 interactions during a type 2 in vivo immune response. J Immunol. 1997;158:4088–96. [PubMed] [Google Scholar]
- 38.Scales HE, Ierna MX, Gutierrez-Ramos JC, Coyle AJ, Garside P, Lawrence CE. Effect of inducible costimulator blockade on the pathological and protective immune responses induced by the gastrointestinal helminth Trichinella spiralis. Eur J Immunol. 2004;34:2854–62. doi: 10.1002/eji.200324364. [DOI] [PubMed] [Google Scholar]
- 39.Kim M-Y, Bekiaris V, McConnell FM, Gaspal FM, Raykundalia C, Lane PJL. OX40 signals during priming on dendritic cells inhibit CD4 T cell proliferation. IL-4 switches off OX40 signals enabling rapid proliferation of Th2 effectors. J Immunol. 2005;174:1433–7. doi: 10.4049/jimmunol.174.3.1433. [DOI] [PubMed] [Google Scholar]
- 40.Ishii N, Ndhlovu LC, Murata K, Sato T, Kamanaka M, Sugamura K. OX40 (CD134) and OX40 ligand interaction plays an adjuvant role during in vivo Th2 responses. Eur J Immunol. 2003;33:2372–81. doi: 10.1002/eji.200324031. [DOI] [PubMed] [Google Scholar]
- 41.Murata K, Nose M, Ndhlovu LC, Sato T, Sugamura K, Ishii N. Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases. J Immunol. 2002;169:4628–36. doi: 10.4049/jimmunol.169.8.4628. [DOI] [PubMed] [Google Scholar]
- 42.Jember AG, Zuberi R, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193:387–92. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecaberg B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 44.Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam R, Kurimoto M, Sur S. IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164:2701–10. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 45.Lawrence CE, Paterson YY, Wright SH, Knight PA, Miller HR. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology. 2004;127:155–65. doi: 10.1053/j.gastro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun. 1983;41:445–7. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–56. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–95. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kashiwakura J, Yokoi H, Saito H, Okayama Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J Immunol. 2004;173:5247–57. doi: 10.4049/jimmunol.173.8.5247. [DOI] [PubMed] [Google Scholar]
- 50.Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–63. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+) CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–51. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 52.Jutel M, Akdis M, Blaser K, Akdis CA. Are regulatory T cells the target of venom immunotherapy? Curr Opin Allergy Clin Immunol. 2005;5:365–9. doi: 10.1097/01.all.0000173784.81024.7a. [DOI] [PubMed] [Google Scholar]
- 53.Malmstrom V, Shipton D, Singh B, Al-Shamkhani A, Puklavec MJ, Barclay AN, Powrie F. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J Immunol. 2001;166:697–81. doi: 10.4049/jimmunol.166.11.6972. [DOI] [PubMed] [Google Scholar]
- 54.MacDonald TT. Effector and regulatory lymphoid cells and cytokines in mucosal sites. Curr Top Microbiol Immunol. 1999;236:113–35. doi: 10.1007/978-3-642-59951-4_7. [DOI] [PubMed] [Google Scholar]
- 55.Hoshino A, Tanaka Y, Akiba H, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol. 2003;33:861–9. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]