Figure 7.
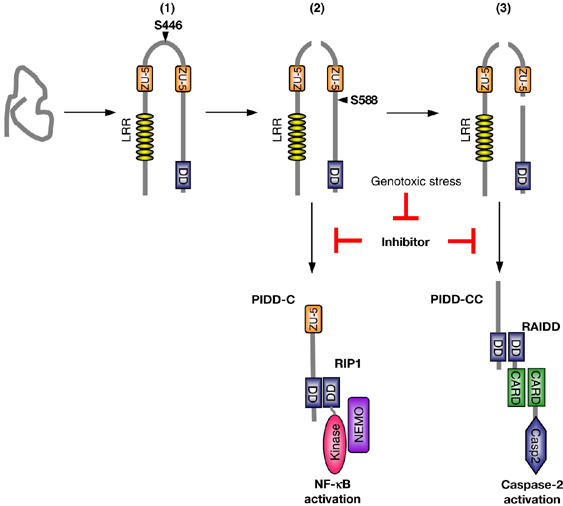
Model of PIDD maturation and its impact on the selection of signaling pathways. Upon synthesis, PIDD folds into a conformation in which one of the potential splicing sites, S446, is immediately active, leading to the generation of PIDD-N and PIDD-C fragments (1). Cleavage at the PIDD-C proteolytic site (2) presumably induces a slight conformational change at the PIDD-CC cleavage site, allowing the proteolytic activity of this site to become active as well, although this second cleavage event appears to be regulated by yet another mechanism, coupled to the severity of the DNA damage (3). The two cleavage events in PIDD lead to the generation of two fragments with distinct functions. Cleavage at S446 generates PIDD-C, which is able to bind RIP1 and NEMO and as such triggers an NF-κB response. Cleavage at S588 generates PIDD-CC, which binds to RAIDD and therefore recruits and activates caspase-2. These two signaling pathways seem to be constitutively inhibited and become activated only upon genotoxic stress.
