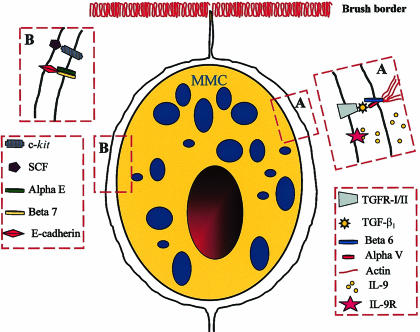
Figure 3.
Diagrammatic representation of a mouse mucosal mast cell (mMMC) within the intestinal epithelium with the postulated receptor–ligand interactions between the two cell types illustrated in boxes A and B. In Box A the epithelial cell-specific integrins αVβ6 are shown binding an activated transforming growth factor-β1 (TGF-β1) molecule and presenting it to its receptor on the mast cell surface. The probable interaction between the integrins and actin fibres in the epithelial cytoskeleton is also illustrated. We speculate that interleukin (IL)-9 is produced by the epithelium, as indicated by published studies,140 and that it binds to its receptor on the mast cell surface. In box B the interaction between epithelially expressed stem cell factor (SCF) and its tyrosine kinase receptor c-kit is shown together with the probable interaction between the integrins αEβ7 on the mast cell surface and epithelially expressed E-cadherin. The receptor–ligand interactions illustrated here are consistent with in vitro studies showing that IL-9, SCF and TGF-β1 are important growth and differentiation factors. We speculate that the constitutive secretion of mouse mast cell proteinase-1 (mMCP-1), induced by TGF-β1,134 exerts a modulatory effect on these receptor–ligand interactions through, for example, the proteolytic degradation of ligands such as SCF or of the cytokines in the intercellular milieu. This hypothesis might explain the augmented mast cell hyperplasia in mMCP-1−/− mice lacking this proteinase.14,19