Abstract
The Fas/Fas ligand (FasL) system plays important roles in the immune system, including host immunoregulation and cytotoxicity. In this study, we investigated the involvement of Fas–FasL interactions in spontaneous acceptance of hepatic allografts in murine orthotopic liver transplantation. Liver transplantation between the C57BL/6 (B6, H-2b) donor and the MRL/Mp (MRL, H-2k) recipient was performed in various combinations of donor and recipient mice with wild type (+/+), Fas-mutant (lpr) or FasL-mutant (gld) genotypes. The prolongation and spontaneous acceptance of the fully allogeneic grafts in recipients was not observed in either MRL-lpr recipients with B6+/+ livers or MRL+/+ recipients with B6-gld livers. Moreover, the serum alanine aminotransferase (ALT) levels and the degree of cell infiltration into hepatic allografts on day 7 after transplantation were inversely correlated with the recipient survival time (in days). The donor-specific cytotoxic T-lymphocyte (CTL) activities of the graft-infiltrating cells (GICs) from MRL-gld recipients with B6+/+ livers were much lower than those from MRL+/+ or -lpr recipients on days 5 and 10 after transplantation. However, the CTL activities of the GICs from MRL+/+ and -gld recipients predominately disappeared by day 15 after transplantation. Furthermore, the anti-donor CTL activities induced in MRL+/+ recipients were ascribed to CD8+ cells, and were not mediated by Fas–FasL interactions. These results strongly suggest that the Fas/FasL system plays a critical role for recipient immunoregulation, enabling recipients in accepting hepatic allografts by deletion of the donor-specific T cells, but not for CTL/target cell interaction in MRL+/+ recipients.
Introduction
Engagement of Fas by Fas ligand (FasL) results in the apoptotic death of Fas-bearing cells.1–3 The Fas antigen expressed on hepatocytes plays an important role in liver diseases, including viral hepatitis, fulminant hepatitis and alcoholic cirrhosis.4–6 In a murine model, administration of an anti-Fas monoclonal antibody (mAb) (Jo2) induces lethal liver damage caused by hepatocyte apoptosis.7 T-cell-mediated cytotoxicity is one of the most critical and fundamental effector mechanisms of allogeneic target cell death. Previous studies have delineated that cytotoxicity is mediated through several major molecular pathways, principally the perforin/granzyme-mediated and/or the Fas–FasL-mediated pathways.8–10 However, their precise roles in hepatic allograft rejection have not yet been determined.
Interestingly, it has been shown that a transplanted, fully allogeneic liver is often spontaneously accepted across a major histocompatibility barrier in mice.11 Murine liver allografts revealed that prominent apoptotic cells were dispersed throughout the non-parenchymal cell population, implying that T-cell deletion may be responsible for spontaneous liver allograft acceptance.12 The underlying mechanism remains to be elucidated in detail. On the other hand, in the case of rat liver transplantation, hepatic allografts can be spontaneously accepted only in limited combinations (e.g. DA→PVG), although the recipients showed transient rejection after transplantation.13 In the rat hepatic allografts, the expression of FasL gradually switched from graft-infiltrating cells (GIC) to hepatocytes when the rejection was naturally overcome and tolerance was induced.14 Therefore, Fas/FasL pathways may contribute to the control of the immune response, but there is no direct evidence to demonstrate the idea that the Fas/FasL system is involved in the induction of hepatic allograft tolerance. It has also been reported that Fas–FasL interaction mediates liver injury during rat hepatic allograft rejection.15
Only in mice are mutations of Fas (lpr) or FasL (gld) available. These mutated mice develop massive lymphadenopathy and are susceptible to autoimmune disease as a consequence of disrupted T-cell homeostasis.16 The activation-induced cell death of mature T cells does not occur in lpr or gld mice in vivo.17 In the present study, we used these mutant mice to clarify whether the Fas–FasL system is involved in recipient immune regulation through activation-induced cell death and spontaneous acceptance/rejection of fully allogeneic hepatic grafts in mice. We also examined whether FasL expression by allograft parenchymal cells or residual donor-derived non-parenchymal cells, such as Kupffer cells, downregulates local immune responses, as observed at the immunologically privileged sites such as in the testis and eye.4,18–20
Materials and Methods
Animals
Male MRL/Mp (MRL, H-2k)-wild type (+/+), lpr/lpr (lpr), gld/gld (gld) and C57BL/6 (B6, H-2b)-lpr mice were purchased from SLC (Shizuoka, Japan). Male B6+/+ and BALB/c (H-2d) mice were bred and maintained in the Institute of Experimental Animals, Kyushu University (Fukuoka, Japan). Male B6-gld mice were purchased from Jackson Laboratory (Bar Harbor, ME). In this study, we used young animals, 5–10 weeks of age, in order to prevent age-dependent dysregulation of the immune system in lpr and gld mutant mice. All mutant mice used in the present study had apparently normal phenotypes (no lymphadenopathy). All animal experiments were conducted in accordance with local institutional guidelines for the care and use of laboratory animals.
Orthotopic liver transplantation
B6 donors into MRL (+/+, lpr, gld) recipients were used. The operations were performed under ether anaesthesia. Livers were transplanted orthotopically, according to Qian et al., with minor modifications.21 This transplantation procedure resulted in indefinite survival for more than 90% of the recipient mice that received syngeneic hepatic grafts. Neither immunosuppressive agents nor antibiotics were used. We used the indicated number of mice in each group for measurement of graft survival. For the measurement of alanine aminotransferase (ALT), histopathological analyses and cytotoxic assays, other recipient mice were used.
ALT assay
ALT levels were measured, using a standard clinical automatic analyser (Hitachi, Tokyo, Japan), in blood samples collected 7 days after grafting. The data from samples with apparent biliary complications were omitted in this study.
Histopathological analyses of GICs and apoptotic cells
Liver samples were fixed in 10% neutral-buffered formalin. Fixed tissues were embedded in paraffin, sections were taken and these sections were stained with haematoxylin and eosin (H & E). GICs in the portal areas of liver grafts were detected by H & E staining. Apoptotic bodies were detected by terminal deoxynucleotidyl-transferase-catalysed deoxyuridine triphosphate-digoxigenine nick-end labelling (TUNEL), performed using the in situ cell death detection kit (Oncor, Gaithersburg, MD), according to the manufacturer's instructions. Active caspase-3+ cells were detected with purified monoclonal rabbit anti-active caspase-3 (PharMingen, San Diego, CA), used according to the manufacturer's instructions. The sections were also counterstained with haematoxylin. The number of TUNEL+ and active caspase-3+ cells per high-power field was counted by microscopy (magnification ×40).
Preparation of GICs
After the mice were killed, hepatic allografts were surgically removed following in situ perfusion with saline and then disrupted using two glass slides. GICs were isolated by centrifugation, using a Percoll gradient (Sigma Chemical Co., Louis, MO). Cells at the 1·086 and 1·055 Percoll interface were recovered and then washed twice with saline. The GIC suspensions in each group were pooled and the number of viable nucleated cells was counted using Trypan Blue dye exclusion. CD4-depleted or CD8-depleted GICs were prepared immunomagnetically using magnetic beads (Dynal, Oslo, Norway) and anti-CD4 (clone H129·19) or anti-CD8 (clone 53-6·7) mAb (PharMingen), according to the manufacturer's instructions.
Flow cytometric analyses
GICs isolated from liver allografts were analysed. After blocking non-specific antibody binding with a mAb specific for the mouse Fc receptor (clone 2·4 G2), the expressions of various cell-surface antigens were analysed by two-colour flow cytometry using a fluorescence-activated cell sorter (FACSCalibur; Becton-Dickinson, Mountain View, CA). The following mAbs were used in this study: fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-H-2Kb (Meiji Institute of Health Science, Tokyo, Japan), anti H-2Kk (Meiji Institute of Health Science), anti-CD3 (clone 145-2C11; PharMingen), anti-CD4 (clone H129·19; PharMingen) and anti-CD8 (clone 53-6·7; PharMingen). The data were acquired and processed using CELLQuest software (Becton-Dickinson).
Cytotoxic assays
Freshly isolated GICs obtained from the B6 hepatic allografts in the MRL recipients were used as effectors. Concanavalin A (Con A)-stimulated spleen cells were used as target cells. Effector cells were incubated with 1 × 104 51Cr-labelled target cells at various effector : target (E : T) ratios in 0·2 ml of complete RPMI-1640 (Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (Gibco BRL, Grand Island, NY) in a 96-well round-bottomed microplate at 37° in humidified air with 5% CO2. After a 4-hr incubation period, every culture supernatant was collected and the radioactivity was measured using an Autowell gamma counter (Aloka, Tokyo, Japan). The % cytotoxicity of specific 51Cr release was calculated using the following formula:
 |
Radioactivities of maximum and spontaneous 51Cr release [in counts per minute (c.p.m.)] were obtained by incubating target cells with 1% Triton-X-100 and with medium alone, respectively. All samples were performed in triplicate.
Statistical analyses
All the data, except survival duration (in days), were presented as means ± standard error of the mean (SEM). They were first analysed for the homogeneity of variance with the F-test. The Student's t-test was used if the group variance appeared homogeneous, and Welch's t-test was used if the variance was not homogeneous. The numbers of days that the grafts survived were compared using Kaplan-Meier analysis and the log-rank test. A P-value of less than 0·05 was considered statistically significant.
Results
Survival of recipient mice after liver transplantation
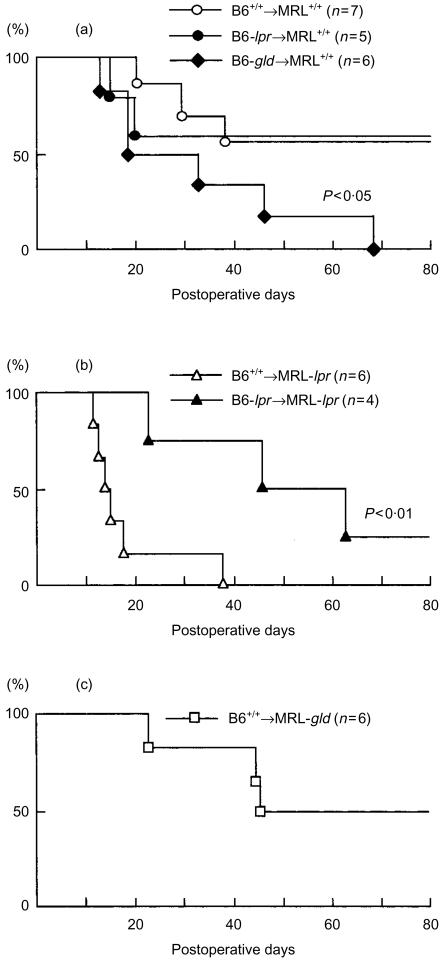
In order to determine the contribution of the Fas–FasL system to recipient survival, B6-derived hepatic allografts (+/+, -lpr or -gld) were transplanted to MRL recipients (+/+, -lpr or -gld) in various combinations. The recipient's survival was checked daily after liver transplantation. As shown in Fig. 1(a), in the case of MRL+/+ recipients grafted with B6+/+ donor livers, the recipients experienced relatively long-term survival (median survival >80 days) and four of the seven recipients survived for longer than 80 days. The median survival time of MRL+/+ recipients grafted with B6-lpr donor livers (> 80 days) was comparable to that of MRL+/+ recipients with B6+/+ grafts. In contrast, MRL+/+ recipients with B6-gld grafts died significantly earlier (median survival 32 days) than MRL+/+ recipients with B6+/+ grafts (P < 0·05). MRL-lpr recipients grafted with B6+/+ donor livers appeared systemically ill (weight loss and mild lethargy) and died shortly after transplantation (median survival 14·5 days), whereas MRL-lpr recipients with B6-lpr grafts generally appeared well and survived for longer (median survival > 54 days) (P < 0·01), as shown in Fig. 1(b). MRL-gld recipients grafted with B6+/+ donor livers experienced long- term survival (median survival > 63 days), as observed in MRL+/+ recipients with B6+/+ grafts (Fig. 1c). Thus, the prolongation and spontaneous acceptance of the fully allogeneic grafts in recipients was not observed in the case of either B6+/+ grafting into MRL-lpr recipients or B6-gld grafting into MRL+/+ recipients. These results strongly suggest that in MRL+/+ recipients, hepatic allograft destruction is not mediated by the Fas–FasL system. Specifically, FasL expression in the grafted liver is relevant to the acceptability of hepatic allograft. By contrast, this research demonstrates that in MRL-lpr recipients, hepatic allograft destruction is partially mediated by the Fas–FasL system.
Figure 1.
Survival of recipient mice after allogeneic liver transplantation. Hepatic allografts from B6+/+, -lpr and -gld donor mice were orthotopically transplanted into MRL+/+ (a), -lpr (b) or -gld (c) recipients. Survival of mice was observed daily after transplantation.
Serum ALT levels in recipients with liver transplants
In order to evaluate animals for possible liver failure after transplantation, serum levels of the cytosolic enzyme, ALT, were determined in recipients on day 7 after transplantation, when no recipient mice had died but some appeared ill. ALT levels were also examined on days 5, 10 and 15 post-transplantation. On day 5, the rejection process was not fully evident, with only a slight increase in the ALT level (data not shown). On days 10 and 15, ALT levels varied too widely (even in the same group) for comparison of the differences between given groups (data not shown). There were salient differences in serum ALT levels on day 7 after transplantation among some combination groups (Table 1). The mean serum ALT level of MRL+/+ recipients grafted with B6-lpr donor livers was comparable to that of MRL+/+ recipients with B6+/+ grafts, although both serum ALT levels were approximately sixfold higher than the baseline MRL+/+ level. By contrast, the serum ALT level of MRL+/+ recipients with B6-gld grafts increased significantly, compared with that of MRL+/+ recipients with B6+/+ grafts (P < 0·05). A dramatic increase in serum ALT level was observed in MRL-lpr recipients with B6+/+ grafts, but not in those with B6-lpr grafts (P < 0·05). The mean serum ALT level of MRL-gld recipients with B6+/+ or -gld grafts was comparable to the non-treated or naive MRL level (baseline). These results revealed that serum ALT levels correlated highly with the recipient survival, equivalent to hepatic allograft rejection.
Table 1.
Serum alanine aminotransferase (ALT) levels on day 7 after liver transplantation
| Combination | ALT (U/l) |
|---|---|
| Naive MRL+/+ | 49 ± 3 |
| B6+/+ into MRL+/+ | 309 ± 52* |
| B6-lpr into MRL+/+ | 309 ± 64 |
| B6-gld into MRL+/+ | 657 ± 151* |
| B6+/+ into MRL-lpr | 1412 ± 271† |
| B6-lpr into MRL-lpr | 388 ± 248† |
| B6+/+ into MRL-gld | 69 ± 9 |
| B6-gld into MRL-gld | 60 ± 13 |
Data represent average ± standard error of the mean (SEM) values obtained from three to five recipient animals.
Significant difference (P < 0·05) between B6 +/+ into MRL+/+ and B6-gld into MRL+/+.
Significant difference (P < 0·05) between B6 +/+ into MRL-lpr and B6-lpr into MRL-lpr.
Histopathological studies
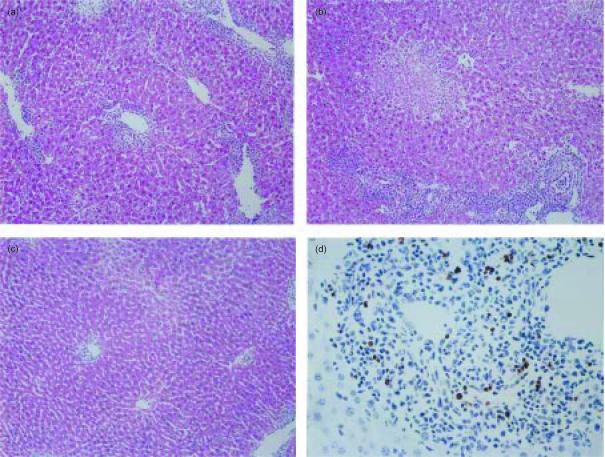
We next examined histopathological differences in the donor livers among the combination groups on day 7 after transplantation. Mild-to-moderate infiltration of mononuclear cells was observed in B6+/+ donor hepatic allografts in MRL+/+ recipients (Fig. 2a). The infiltration was also observed in B6-gld donor hepatic allografts in MRL+/+ recipients (data not shown). B6+/+ donor hepatic allografts in MRL-lpr recipients showed multifocal areas with parenchymal necrosis and massive infiltration of GICs (Fig. 2b), while their severities were ameliorated in B6-lpr donor hepatic allografts (data not shown). On the other hand, B6+/+, as well as B6-gld, donor hepatic allografts in MRL-gld recipients, showed a significantly milder infiltration compared with B6+/+ donor hepatic allografts in MRL+/+ or -lpr recipients (Fig. 2c).
Figure 2.
Analysis of graft-infiltrating cells (GICs) and apoptotic cells on B6+/+ donor hepatic allografts in MRL recipient mice. Sections were prepared from B6+/+ donor livers in MRL+/+ (a), -lpr (b) and -gld (c and d) recipients on day 7 after transplantation. The sections were stained with haematoxylin & eosin (a–c), and apoptotic cells in portal areas of liver grafts were detected by terminal deoxynucleotidyl-transferase-catalysed deoxyuridine triphosphate-digoxigenine nick-end labelling (TUNEL) staining (d). Representative photographs are shown. Original magnification: (a–c), ×10; (d), ×40.
In order to determine whether the Fas–FasL system is a major mechanism of apoptotic cell death in rejecting hepatic allografts, we compared the number of apoptotic cells within rejecting hepatic allografts in MRL+/+, -lpr and -gld recipients on day 7 after transplantation using the TUNEL method. B6+/+, as well as -gld, donor hepatic allografts in MRL+/+ recipients showed that few parenchymal cells (data not shown) and GICs were TUNEL+ (Table 2). FasL expression in the grafted liver did not result in an increase in TUNEL+ GICs. B6+/+ donor hepatic allografts in MRL-lpr recipients showed an increased number of TUNEL+ parenchymal cells in the necrotic areas (data not shown) and a relatively increased number of TUNEL+ GICs beyond expectation. By contrast B6+/+, as well as B6-gld, donor hepatic allografts in MRL-gld recipients had a significantly increased number of TUNEL+ GICs as compared to that in MRL+/+ recipients (Fig. 2d). These results strongly suggest that Fas expression on the GICs is irrelevant to the number of TUNEL+ GICs. To confirm these results in detail regarding apoptosis, we examined active caspase-3+ GICs. Active caspase-3 (CPP32) is required for the induction of apoptosis by certain effectors, such as Fas.22,23 The results of active caspase-3 immunohistochemical examination were similar to that of TUNEL examination (Table 2).
Table 2.
The number of terminal deoxynucleotidyl-transferase-catalysed deoxyuridine triphosphate-digoxigenine nick-end labelling (TUNEL)-positive and active caspase 3-positive graft-infiltrating cells (GICs) on day 7 after liver transplantation
| Combination | TUNEL+ GICs | Active caspase 3+ GICs |
|---|---|---|
| B6+/+ into MRL+/+ | 7·0 ± 0·7* | 4·4 ± 1·2† |
| B6-gld into MRL+/+ | 6·6 ± 1·4 | 4·2 ± 0·4 |
| B6+/+ into MRL-lpr | 9·8 ± 1·5 | 6·4 ± 0·4 |
| B6+/+ into MRL-gld | 15·6 ± 2·9* | 9·2 ± 0·7† |
| B6-gld into MRL-gld | 14·2 ± 1·9 | 8·6 ± 1·4 |
Data represent average ± standard error of the mean (SEM) values obtained from five high-power fields of each slide. Similar results were obtained in two other independent experiments.
Significant difference (P < 0·05) between B6 +/+ into MRL+/+ and B6+/+ into MRL-gld.
Significant difference (P < 0·01) between B6 +/+ into MRL+/+ and B6+/+ into MRL-gld.
Cytotoxic T lymphocyte (CTL) activities of freshly isolated graft-infiltrating cells
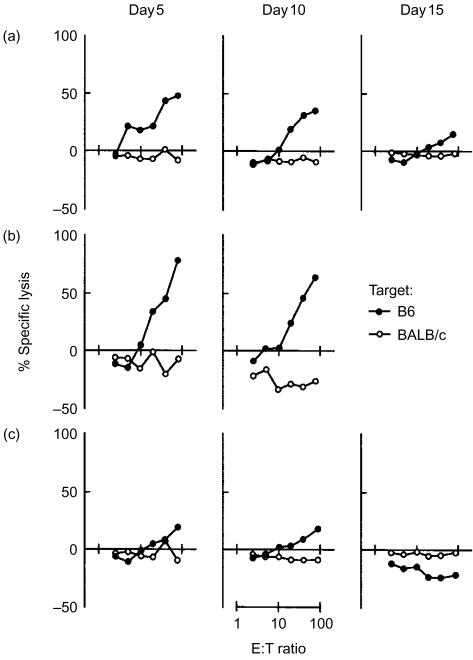
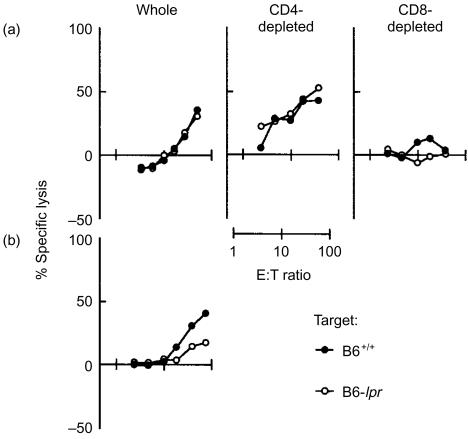
MRL recipients grafted with B6+/+ donor livers were killed on days 5, 10 or 15 after transplantation. Immediately after killing the mice, GICs were isolated as described in the Materials and methods. As reported previously,12 the hepatic allografts were rapidly infiltrated by recipient-derived cells after transplantation. More than 95% of GICs were H-2k-positive (recipient-derived) through the observation period. The proportion of CD4+ CD8+ cells in GICs were very similar among MRL+/+, -lpr and -gld recipient mice. Approximately 20% of GICs were CD4+ and 33% of GICs were CD8+. Few double-negative T cells (CD3+ CD4− CD8−) were found, even in MRL-lpr and -gld recipients (data not shown). As shown in Fig. 3, high CTL activities of GICs against donors, but not against a third party (BALB/c, H-2d), were observed in both MRL+/+ and -lpr recipients grafted with B6+/+ donor livers on days 5 and 10 after transplantation. However, a major part of the CTL activities of GICs from the MRL+/+ recipients disappeared by day 15 after transplantation. By contrast, anti-donor CTL activities of GICs from MRL-gld recipients with B6+/+ livers were much lower than those observed in MRL+/+ and -lpr recipients on days 5 and 10 after transplantation, and they were undetectable on day 15 after transplantation. Although only two data were obtained for CTL activities in lpr recipients on day 15 (when lpr recipient animals were critically ill), the CTL activity persisted as compared to MRL+/+ recipients (data not shown). We next examined whether these CTL activities are dependent on the Fas/FasL system. As shown in Fig. 4(a), the anti-donor CTL activities of GICs from MRL+/+ recipients were independent of the Fas/FasL system, because CTL activities were not depressed against B6-lpr target cells. It was also revealed that CD4+ cell-depleted, but not CD8+ cell-depleted, GICs had the CTL activities in MRL+/+ recipients. By contrast, Fas–FasL-dependent CTL activities were apparent in MRL-lpr recipients, because CTL activities were depressed against B6-lpr target cells, as shown in Fig. 4(b).
Figure 3.
Cytotoxic T-lymphocyte (CTL) activities of graft-infiltrating cells (GICs) in B6+/+ hepatic allografts in MRL recipient mice. GICs were recovered from B6+/+ donor livers in MRL+/+ (a), -lpr (b) and -gld (c) recipients on days 5, 10 and 15 after transplantation. A short-term 4-hr 51Cr-release assay was performed to determine the cytotoxicities of GICs against B6 and BALB/c splenic concanavalin A (Con A) blast cells. Data represent average values from triplicate wells. Similar results were obtained in two other independent experiments. E:T ratio, effector-to-target ratio.
Figure 4.
Cytotoxic T-lymphocyte (CTL) activities, 5 days post-transplantation, of graft-infiltrating cells (GICs) in B6+/+ hepatic allografts in MRL+/+ (a) and -lpr (b) recipients, grafted with B6+/+ donor livers. A short-term 4-hr 51Cr-release assay was performed to determine the cytotoxicities of whole, CD4-depleted and CD8-depleted GICs against B6+/+ (Fas+) and -lpr (Fas−) splenic concanavalin A (Con A) blast cells. Data represent average values from triplicate wells. Similar results were obtained in two other independent experiments.
Discussion
Perforin/granzyme- and Fas/FasL-based mechanisms may account for all T-cell-mediated cytotoxicity in short-term in vitro assays.8 In normal mice, in vivo injection of anti-Fas mAb leads to massive apoptosis in the liver, with death of the animal following within a few hours as a result of fulminant hepatitis because hepatocytes, which express Fas antigen, are highly sensitive to Fas-mediated apoptosis.7 In one aspect of the present study, we examined whether Fas–FasL interaction (CTL/target liver cell interaction) is a major pathway of hepatic allograft destruction. Previous studies have indicated that Fas–FasL interactions may not be essential mediators of T-cell-mediated allograft damage in murine cardiac transplantation.24–26 However, as reported many times previously, Fas and FasL are expressed in various cells, such as hepatocytes, Kupffer cells and lymphocytes, which are involved in hepatic allograft rejection. We also examined these expressions in hepatic allografts using FACS analysis and immunohistochemistory (data not shown). Fas and FasL were also expressed in so many different types of cells in our examination that elucidating the Fas and FasL involvement in the hepatic allograft was difficult. However, it is conceivable that both Fas and FasL are involved in hepatic allograft rejection. Therefore, we studied the involvement of Fas and FasL in hepatic allograft rejection, using various donor–recipient combinations of wild type (+/+), lpr and gld mice.
In MRL+/+ recipients, Fas–FasL interactions are not absolutely required for hepatic allograft destruction, as evidenced by the lack of apparent improvement of recipient survival and ALT data by grafting B6-lpr livers and by generation of Fas/FasL-independent donor-specific cytotoxicities of GICs. These results strongly suggest that the perforin/granzyme pathway is one of the major pathways of T-cell-mediated cytotoxicity in murine hepatic allograft rejection in the case of MRL+/+ recipients, and only CD8+ T cells are responsible for the donor-specific cytotoxicity. Although apoptosis has been reported as a mode of hepatocyte death in allograft rejection,27 the Fas/FasL system hardly affected this apoptotic process of hepatocytes in MRL+/+ recipients, as shown in the present study.
By contrast, in the MRL-lpr recipients, an involvement of the Fas/FasL system in severe hepatic allograft destruction was apparent because it was ameliorated by grafting B6-lpr livers into the recipients. In fact, lpr mouse-derived T cells scarcely express Fas antigen, but overexpress FasL antigen.28 Additionally, Russel et al.17 have reported that in vivo activation-induced cell death of mature T cells does not occur in lpr or gld mice. The emergence of Fas/FasL-mediated cytotoxicity in the MRL-lpr recipient is probably the result of repeated antigenic stimulation of T cells that cannot undergo apoptosis through the Fas-mediated pathway. However, the number of TUNEL-positive GICs increased in the MRL-lpr recipients more significantly than anticipated, as compared with that of the MRL+/+ recipients. Desbarats et al.29 demonstrated that FasL itself can transduce signals leading to cell-cycle arrest and cell death in CD4+ T cells. Thus, interactions between FasL-bearing recipient GICs and Fas expressed in hepatic allografts may increase the number of TUNEL+ GICs. It is also possible that other death signals play important roles in the apoptotic process of GICs in lpr recipients.
Because, according to previous reports,16 in vivo activation-induced cell death of mature T cells may not occur in gld recipients, there may be few apoptotic T cells with the capacity of anti-donor cytotoxicity (which normally results in severe rejection). However, in the MRL-gld recipients, the increased number of apoptotic GICs was associated with a milder infiltration of cells into the hepatic allografts, lower serum ALT levels and lower anti-donor CTL activities on postoperative day 15, in comparison to those observed in the MRL+/+ or -lpr recipients. Wagener et al.30 also demonstrated that heart allografts can be accepted without T-cell apoptosis mediated by the Fas–FasL interactions using gld recipients. One possible explanation for the abrogation of CTL function in gld recipients might be a hyporesponsiveness of T cells from gld mice towards the alloantigens. We examined in vitro CTL induction using spleen cells from gld mice, but the induced CTL activities were comparable to those from the wild-type mice (data not shown). The results indicate that the abrogation of CTL function in the gld recipients is not a result of their hyporesponsiveness to the donor alloantigens. Therefore, a more plausible explanation is that the lower serum ALT levels and the abrogation of CTL function in MRL-gld recipients are attributable to the massive apoptosis of GICs, as shown in Table 2 and Fig. 2(d). Moreover, the increased number of GICs may not be a result of the interaction between Fas-expressing GICs and FasL-bearing cells in the graft because the number of apoptotic GICs was not significantly decreased in the B6-gld liver allografts (Table 2). The precise mechanism that accounts for these results is controversial. However, it has been reported that the Fas–FasL interaction resulted in the release of proinflammatory cytokines such as interleukin (IL)-1β.31 FasL transgenic mice with FasL expression in pancreatic β cells under the control of the insulin promotor underwent destruction of the β cells and consequent diabetes as a result of massive neutrophil recruitment. This neutrophilic response may be responsible for the direct action of FasL itself or may be mediated by a neutrophilic cytokine, IL-8.32 The lack of Fas–FasL engagement and the resultant release of proinflammatory cytokines may lead to accelerated apoptosis and restricted infiltration of GICs.
FasL is a highly efficient mediator of apoptosis in activated T lymphocytes, thus creating an immunoprivileged site, much like the anterior chamber of the eye and Sertoli cells of testis.19,20 Testis grafts, derived from mice that can express functional FasL, survived indefinitely when transplanted under the kidney capsule of allogeneic animals, whereas testis grafts derived from mutant gld mice were rejected.20 Li et al.33 have reported prolonged survival of rat liver allografts transfected with FasL-expressing plasmids. FasL mRNA was found within hepatocytes in an inflammatory liver disease.4 The interaction between FasL on hepatic allografts and Fas on GICs may provide a mechanism that underlies apoptosis of GICs. We next examined whether FasL expression by allograft parenchymal cells4 or residual donor-derived non-parenchymal cells, such as Kupffer cells,18 creates an immunologically privileged status such as testis and eye, comparing the data from B6+/+ donor livers with that of B6-gld donor livers. In the present study, MRL+/+ recipients grafted with B6-gld hepatic allografts died significantly earlier than MRL+/+ recipients with B6+/+ hepatic allografts. Additionally, serum ALT levels of MRL+/+ recipients with B6-gld donor livers significantly increased as compared with those of MRL+/+ recipients with B6+/+ donor livers. However, the histological differences on day 7 post-transplantation were not apparent between B6+/+ and -gld donor hepatic allografts in MRL+/+ recipients. The involvement of antibody, complement, cytokines, etc., may result in these discrepancies. Moreover, the number of TUNEL+ GICs in B6+/+ hepatic allografts in MRL+/+ recipients was comparable to that in B6-gld hepatic allografts in MRL+/+ recipients. These results indicate that FasL expression in hepatic allografts per se cannot create an immunoprivileged site. On the other hand, George et al.34 demonstrated that expression of functional FasL on donor bone marrow cells can induce tolerance induction. Hepatic allografts have a relatively large number of passenger leucocytes35 and probably contain pluripotent stem cells, such as bone marrow.36 It is feasible that FasL-bearing passenger leucocytes or stem cells play an important role in the acceptability of hepatic allografts. Despite our study, the mechanism by which the donor-reactive T cells were deleted remains controversial. No lymphatic vessels could be reconstructed within the grafted livers in this system. However, the possibility that the donor APCs migrate to the lymphoid tissues and, thereafter, that the apoptosis of the recipient T cells is induced, cannot be excluded.
Conclusively, the Fas–FasL system plays a critical role in the spontaneous acceptability of hepatic allografts by deleting donor-specific CTLs, but not for CTL–target cell interactions in MRL+/+ recipients. Thus, Fas–FasL interactions are involved in the early phase of tolerance induction in murine liver transplantation. The question remains as to why hepatic allografts are readily accepted in mice compared with other animals.
Acknowledgments
This work was supported by Health Sciences Research Grants (Research on Immunology, Allergy and Organ Transplantation) from Ministry of Health and Welfare, Japan.
Abbreviations
- ALT
alanine aminotransferase
- Con A
concanavalin A
- c.p.m.
counts per minute
- E:T
effector to target (ratio)
- FasL
Fas ligand
- FITC
fluorescein isothiocyanate
- GICs
graft-infiltrating cells
- H & E
haematoxylin & eosin
- mAb
monoclonal antibody
- PE
phycoerythrin
- SEM
standard error of the mean
- TUNEL
terminal deoxynucleotidyl-transferase-catalysed deoxyuridine triphosphate-digoxigenine nick-end labelling
References
- 1.Dhein J, Walczak H, Bäumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 2.Brunner T, Mogil RJ, LaFace D, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–4. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 3.Ju ST, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 4.Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–30. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–13. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 6.Seino K, Kayagaki N, Takeda K, Fukao K, Okumura K, Yagita H. Contribution of Fas ligand to T cell-mediated hepatic injury in mice. Gastroenterology. 1997;113:1315–22. doi: 10.1053/gast.1997.v113.pm9322527. [DOI] [PubMed] [Google Scholar]
- 7.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–9. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 8.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Goldstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–30. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 9.Kojima H, Shinohara N, Hanaoka S, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–64. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 10.Lowin B, Hahne M, Mattman C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–2. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 11.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–24. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, Fung JJ, Thomson AW. Apoptosis within spontaneously accepted mouse liver allografts. J Immunol. 1997;158:4654–61. [PMC free article] [PubMed] [Google Scholar]
- 13.Kamada N, Brons G, Davies HS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429–31. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Pan TL, Goto S, Lin YC, et al. The Fas and Fas ligand pathways in liver allograft tolerance. Clin Exp Immunol. 1999;118:180–7. doi: 10.1046/j.1365-2249.1999.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiroyasu S, Shiraishi M, Koji T, Mamadi T, Sugawa H, Muto Y. Analysis of the Fas system and Bcl-2 in rat liver allograft rejection. J Surg Res. 1999;84:204–11. doi: 10.1006/jsre.1999.5644. [DOI] [PubMed] [Google Scholar]
- 16.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 17.Russel JH, Rush B, Weaver C, Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA. 1993;90:4409–13. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müschen M, Warskulat U, Peters-Regehr T, Bode JG, Kubitz R, Häussinger D. Involvement of CD95 (APO-1/Fas) ligand expressed by rat Kupffer cells in hepatic immunoregulation. Gastroenterology. 1999;116:666–77. doi: 10.1016/s0016-5085(99)70189-7. [DOI] [PubMed] [Google Scholar]
- 19.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 20.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 21.Qian S, Fung JJ, Demetris AJ, Ildstad S, Starzl TE. Orthotopic liver transplantation in mice. Transplantation. 1991;52:562–4. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen CP, Alexander DZ, Hendrix R, Ritchie SC, Peason TC. Fas-mediated cytotoxicity. Transplantation. 1995;60:221–4. doi: 10.1097/00007890-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 24.Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–6. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 25.Seino K, Kayagaki N, Bashuda H, Okumura K, Yagita H. Contribution of Fas ligand to cardiac allograft rejection. Int Immunol. 1996;8:1347–54. doi: 10.1093/intimm/8.9.1347. [DOI] [PubMed] [Google Scholar]
- 26.Djamali A, Odorico JS. Fas-mediated cytotoxicity is not required for rejection of murine nonvascularized heterotopic cardiac allografts. Transplantation. 1998;66:1793–801. doi: 10.1097/00007890-199812270-00038. [DOI] [PubMed] [Google Scholar]
- 27.Krams SM, Egawa H, Quinn MB, Villanueva JC, Garcia-Kennedy R, Martinez OM. Apoptosis as a mechanism of cell death in liver allograft rejection. Transplantation. 1995;59:621–5. [PubMed] [Google Scholar]
- 28.Chu JL, Ramos P, Rosendorff A, Nikolic-Zugic J, Lacy E, Matsuzawa A, Elkon KB. Massive up-regulation of the Fas ligand in lpr and gld mice: implications for Fas regulation and the graft-versus-host disease-like wasting syndrome. J Exp Med. 1995;181:393–8. doi: 10.1084/jem.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desbarats J, Duke RC, Newell MK. Newly discovered role for Fas ligand in the cell-cycle arrest of CD4+ T cells. Nat Med. 1998;4:1377–82. doi: 10.1038/3965. [DOI] [PubMed] [Google Scholar]
- 30.Wagener ME, Konieczny BT, Dai Z, Ring GH, Lakkis FG. Alloantigen-driven T cell death mediated by Fas ligand and tumor necrosis factor-α is not essential for the induction of allograft acceptance. Transplantation. 2000;69:2428–32. doi: 10.1097/00007890-200006150-00037. [DOI] [PubMed] [Google Scholar]
- 31.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase-1 independent IL-1β release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–92. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 32.Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, Stock PG, Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997;3:738–43. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 33.Li XK, Okuyama T, Tamura A, et al. Prolonged survival of rat liver allografts transfected with Fas ligand-expressing plasmid. Transplantation. 1998;66:1416–23. doi: 10.1097/00007890-199812150-00003. [DOI] [PubMed] [Google Scholar]
- 34.George JF, Sweeney SD, Kirklin JK, Simpson EM, Goldstein DR, Thomas JM. An essential role for Fas ligand in transplantation tolerance induced by donor bone marrow. Nat Med. 1998;4:333–5. doi: 10.1038/nm0398-333. [DOI] [PubMed] [Google Scholar]
- 35.Sun J, Sheil AG, Wang C, et al. Tolerance to rat liver allografts. IV. Acceptance depends on the quantity of donor tissue and on donor leukocytes. Transplantation. 1996;62:1725–30. doi: 10.1097/00007890-199612270-00005. [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]