Abstract
The mucosal adjuvant properties of the three type 2 ribosome-inactivating proteins (RIPs) from the European mistletoe, Viscum album L., were investigated. Mistletoe lectins were compared with cholera toxin (CT) as adjuvants when delivered nasotracheally together with herpes simplex virus glycoprotein D2 (gD2). All three mistletoe lectins (MLI, MLII, MLIII) were potent mucosal adjuvants. Co-administration of MLI, MLII or MLIII with gD2 led to significantly higher levels of gD2-specific mucosal immunoglobulin A (IgA) and systemic immunoglobulin G (IgG) antibody than when the antigen was delivered alone. The levels of antibodies induced were similar to those generated in mice immunized with gD2 and the potent mucosal adjuvant CT. Administration of ML1 with gD2 enhanced the antigen-specific splenic T-cell proliferative response. Interleukin-5 (IL-5), but not interferon-γ (IFN-γ), was detected in supernatants from splenocytes stimulated in vitro with gD2. This indicates that MLI enhanced type 2 T-helper cell (Th2) responses to the bystander antigen, gD2. Analysis of the gD2- and lectin-specific IgG subclass titres in mice immunized with gD2 and MLI, MLII or MLIII revealed a high ratio of IgG1 : IgG2a, which is compatible with the selective induction of Th2-type immune responses.
Introduction
Relatively few molecules have been identified that induce strong immune responses when delivered alone by mucosal routes. This is attributed to physiochemical barriers at the mucosae and to aspects of the mucosal immune system. However, some molecules are highly immunogenic when delivered alone by oral, nasotracheal or other mucosal routes. Most of these are proteins/glycoproteins of the lectin/toxin families, the most potent and well characterized of which are the bacterial A-B toxins. It has been suggested that lectin-like properties confer mucosal immunogenicity on proteins.1 Conjugation of the non-immunogenic hapten, trinitrophenyl (TNP) to any of a number of plant lectins has been shown to lead to a significantly higher hapten-specific antibody response following oral delivery to mice. However, recent work showed that not all plant lectins were strong immunogens in mice when delivered by the oral or nasotracheal routes.2 Rather, there was a wide spectrum of immunogenicity, with lectins such as wheatgerm agglutinin (WGA, Triticum aestivum) and UEA-1 (Ulex europaeus agglutinin 1) being poor immunogens. In contrast, tomato agglutinin (LEA, Lycopersicum esculentum) and particularly mistletoe lectin I (MLI, Viscum album) were strong immunogens. It was further shown that when co-administered with a bystander antigen (ovalbumin) by the nasotracheal route, MLI was as effective as cholera toxin (CT) as a mucosal adjuvant.3 A number of other plant lectins were tested, but none resulted in a significantly enhanced response compared to the antigen alone.
ML1 belongs to a class of plant lectins called ribosome-inactivating proteins (RIPs). Many higher plants produce RIPs, defined as plant proteins that can catalytically inactivate eukaryotic ribosomes,4,5 by the removal of an adenine residue from the 28S ribosomal RNA (rRNA). Recent evidence indicates that RIPs can not only deadenylate rRNA but can also use other polynucleotides as a substrate and may cleave multiple adenosine residues from a single substrate molecule. RIPs can thus be termed polynucleotide-adenosine-glycosidases.6 RIPs can be subdivided into three groups based on structure. Type 1 RIPs consist of a catalytically active single-subunit protein chain of 25 000–30 000 molecular weight (MW). Type 3 RIPs have a similar structure to type 1 RIPs but the intact polypeptide (around 30 000 MW) acts as a zymogen which is converted into the enzymatically active form after post-translational processing. The mature type 3 RIPs consist of an α- and a β-chain, both derived from a single precursor.7 Type 2 RIPs are composed of one or more protomers consisting of two different disulphide bridge-linked A- and B-chains.8 The A-chain has N-glycosidase activity and the B-chain has carbohydrate-binding domains. Because of this carbohydrate-binding property, type 2 RIPs are considered as lectins and specifically as chimerolectins because they possess both enzymatic and lectin activity.9 All type 2 RIPs cloned to date (including MLI, MLII and MLIII) share a high degree of sequence homology and have similar conformation. The MLI B-chain mediates binding to carbohydrates (galactose),10,11 while the A-chain catalyses the hydrolysis of the N-glycosidic bond at adenine-4324 in the eukaryotic 28S ribosomal RNA. As a result, the 60S subunit cannot bind elongation factor 2, resulting in inhibition of the elongation step of protein biosynthesis.12 MLI is composed of two non-covalently associated pairs of disulphide-linked A-B dimers. Disulphide bond reduction and subunit dissociation are required for cytotoxic activity.13 Variant forms of the lectin, termed MLII and MLIII, are also present in preparations from mistletoe plants.14–16 The molecules can be distinguished from each other by their degree of glycosylation, which occurs during post-translational processing or during the processing of mistletoe preparations.17 At present the principal interest in mistletoe lectins is in alternative therapies for cancer and as components of immunotoxins (a protein toxin attached to a binding ligand such as an antibody). These molecules bind to cell-surface antigens and kill cells by catalytic inhibition of protein synthesis in the cytosol. Immunotoxins have been tested clinically in haematological malignancies and solid tumors, and have demonstrated clinical efficacy.18,19
The hypothesis behind this study was that different type II RIPs from V. album have the ability to enhance immune responses to co-administered protein antigens. The vaccine antigen herpes simplex virus glycoprotein D2 (gD2) was administered with the lectins by the nasotracheal route, and systemic and mucosal immune responses were measured.
Materials and methods
Animals
Female BALB/c mice were obtained from Harlan Olac (Bicester, UK) and used at 8 weeks of age. Animals were given free access to commercial stock diet (Labsure, Manea, UK) and water.
Immunomodulators and antigens
CT was obtained from Sigma (Poole, UK). MLI, MLII and MLIII were prepared from V. album L., as described previously.14 The purity of the mistletoe lectin preparations was 98% (MLI, MLIII) and 96% (MLII), as determined by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and enzyme-linked immunosorbent assay (ELISA). The remainder of the preparations was constituted by the other lectins. Using the Limulus amoebocyte assay (Sigma) the levels of endotoxin contamination in the mistletoe lectin preparations were determined to be less than 0·1 IU/ml (ML1) and less than 0·01 IU/ml (MLII, MLIII). This corresponds to less than 1 pg and 0·1 pg of lipopolysaccharide (LPS), respectively, delivered per mouse. Herpes simplex virus gD2 was provided by the Chiron Corporation (Emeryville, CA). Endotoxin was undetectable in the gD2 preparation using the Limulus assay.
Mucosal immunization
Groups of mice (n = 10) were immunized on days 1, 14, 28 and 42, with phosphate-buffered saline (PBS), gD2 (5 µg) alone, or gD2 (5 µg) mixed with 1 µg of CT, MLI, MLII or MLIII. While restrained, mice were dosed with 30 µl of each preparation (15 µl placed over each nostril) using fine tips attached to a pipette. Mice were held in place until the liquid was inhaled and were not anaesthetized for the procedure. For the experiments on T-cell proliferation and cytokine responses, mice were immunized on days 1, 21 and 42 and samples were taken on day 56.
Collection of blood and mucosal secretions
One day before each immunization, blood samples were collected from the tail vein. Two weeks after the final immunization, animals were terminally anaesthetized (hypnorm plus diazepam) to allow collection of salivary and vaginal secretions. Mice were then killed by anaesthetic overdose followed by exsanguination, and blood was collected and the serum stored at − 20°. Mucosal secretions were collected as described previously.3 Absorbent cellulose wicks (Whatman International, Maidstone, UK) were used to collect saliva and vaginal fluid. Wash fluid [0·01 m PBS, 50 mm EDTA, 5 mm phenylmethylsulphonyl fluoride (PMSF), 5 µg/ml aprotinin (Sigma) (ice-cold)] was used for elution of antibody from wicks and for nasotracheal and intestinal washes. Saliva was collected by the insertion of a wick tip into the mouth for 2 min and antibody extracted into 400 µl of mucosal wash fluid. Vaginal fluid was collected by repeated flushing and aspiration of 50 µl of wash fluid followed by insertion of a wick for 2 min. Antibody was extracted into 400 µl of wash fluid. Nasotracheal washes were collected from decapitated animals by back-flushing 0·5 ml of wash fluid from the trachea. Intestinal washes were obtained by flushing the small intestine with 10 ml of ice-cold wash fluid.
Antibody ELISA
Microtitre plates (Immunolon 4; Dynex Technologies, Chantilly, VA) were coated with 75 µl of antigen per well (1 µg/ml for CT/lectins, 2 µg/ml for gD2) in carbonate-bicarbonate buffer, pH 9·6, and incubated at 4° overnight. After washing, plates were blocked with 2% gelatin/dilution buffer and incubated at 37° for 1 hr. Plates were washed, samples added, serially diluted and incubated at 37° for 1 hr. Biotinylated antiserum in dilution buffer was added and incubated at 37° for 1 hr. Working dilutions of anti-immunoglobulin G (IgG) (1 : 8000) and anti-immunoglobulin A (IgA) (1 : 2600) antibodies (Sigma) were determined after preliminary assays using preimmune and pooled positive sera. Working dilutions of IgG subclass antisera (BD Pharmingen, San Diego, CA) were as recommended by the suppliers (1 : 10 000). ELISA dilution buffers were as follows: CT, PBS + 0·1% Tween (PBST); gD2, PBST; and mistletoe lectins, 100 mm D-galactose in PBST. Sugars were included in dilution buffers to prevent sugar-mediated interactions between immunoglobulins and lectins.2,3 After further washes, ExtrAvidin® peroxidase (Sigma), at a dilution of 1 : 750 in dilution buffer, was added and incubated at 37° for 30 min. Plates were washed and 50 µl/well of developing solution [TMB microwell peroxidase substrate (1-C); Kirkegaard and Perry Laboratories, Gaithersburg, MD] was added and incubated in the dark with shaking at 37° for 30 min. The reaction was stopped by the addition of 1 m H2SO4 and the absorbance read at 450 nm. End-point titres were determined as the dilution of a sample giving an absorbance value of 0·1 units + 1 SD greater than the mean of control samples at the same dilution.
T-cell proliferation and cytokine assays
Spleen and cervical lymph node cells (2 × 106 cells/ml) were cultured in complete RPMI at 37° and 5% CO2 with gD2 or phorbol 12-myristate 13-acetate (PMA, Sigma) (20 ng/ml) and anti-mouse CD3 (Pharmingen) (1 µg/ml). Supernatants were collected after 72 hr for cytokine assays, supernatants were replaced with fresh medium and 3H-thymidine was added to the cells and incubated at 37° for 4 hr. Cells were harvested and thymidine incorporation was determined by scintillation counting. The concentrations of interleukin-5 (IL-5) and interferon-γ (IFN-γ) in supernatants were determined by ELISA.
Statistics
Antibody data are presented as arithmetic means. Analysis of variance (anova) was used to test for significance between groups. Where standard deviations were significantly different between groups, a non-paramentic test (Kruskal–Wallis test with Dunn's multiple comparison post test) was used to assess significance.
Results
We have previously demonstrated that MLI is a strong mucosal adjuvant. To determine if mistletoe lectins II and III share this property we tested their potential to enhance immune responses to co-administered herpes simplex virus gD2.
Serum antibody responses
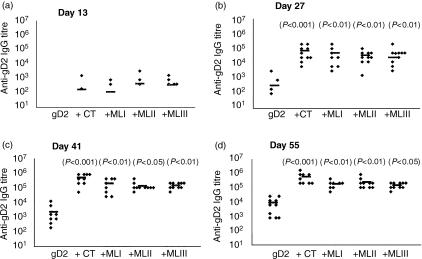
All three mistletoe lectins (MLI, MLII and MLIII) significantly enhanced the levels of serum IgG elicited to co-administered gD2 (Fig. 1). After a single dose, gD2-specific IgG was not detected in sera from mice administered with the antigen alone but was detected in a number of mice immunized with the antigen together with CT (two of 10), MLI (two of 10), MLII (three of 10) and MLIII (five of 10). Two weeks after a second immunization, specific antibody was detected in all mice immunized with gD2 and CT or mistletoe lectins. After the second, third and fourth immunizations with gD2 and lectins, the levels of specific antibody were significantly higher than in the mice that received antigen alone. The titres of antigen-specific antibody were not significantly different between the different mistletoe lectin and CT groups. High levels of lectin/cholera toxin-specific serum IgG were also detected (Table 1). In all cases, specific antibody was detected after a single dose and the titre increased with each subsequent dose (only data from the final time-point is presented).
Figure 1.
Mistletoe lectins (ML) enhance serum immunoglobulin G (IgG) responses to co-administered glycoprotein D2 (gD2) when delivered via the nasotracheal route. The data show gD2-specific serum IgG antibody titres in mice immunized on days 1, 14, 28 and 49 with either gD2 (5 µg) alone or gD2 (5 µg) together with cholera toxin (CT) (1 µg), MLI (1 µg), MLII (1 µg) or MLIII (1 µg). Sera were collected 1 day before each immunization and at the termination of the study. (a) Serum IgG titres after one dose (day 13); (b) serum IgG titres after two doses (day 27); (c) serum IgG titres after three doses (day 41); and (d) serum IgG titres after the final dose (day 55). The diamond symbols represent antibody titres in individual animals and the symbol (–) represents the mean titre. P-values in parentheses refer to significance of data compared with the gD2-only group.
Table 1.
Misletoe lectins I, II and III (MLI, MLII, MLIII) are strong mucosal immunogens
| Serum IgG and IgG subclass titre | IgA titre | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lectin/toxin | IgG | IgG1 | IgG2a | IgG2b | IgG3 | Serum | Saliva | Vagina | Nasal | Gut |
| MLI | 313 600 | 742 400 | 2200 | 18 400 | – | 2700 | 53 | 84 | 432 | 6 |
| MLII | 655 360 | 655 360 | 3040 | 23 040 | – | 520 | 98 | 34 | 166 | 30 |
| MLIII | 179 200 | 327 680 | 120 | 9920 | – | 520 | 58 | 88 | 113 | 7 |
| CT | 523 378 | 568 889 | 176 356 | 193 422 | 211 | 7111 | 484 | 409 | 1771 | 53 |
Data represent the mean lectin/toxin-specific antibody titres in mice immunized nasotracheally with glycoprotein D2 (gD2) (5 µg). Mice (n = 10) were immunized on days 0, 14, 28, 42 and samples were collected on day 56.
CT, cholera toxin; IgG, immunoglobulin G.
One of 10 animals in the CT + gD2 group (following the second immunization) and two of 10 animals in the MLI + gD2 group (following the first immunization) were killed as a result of their poor condition. Mice in all three of the mistletoe lectin groups suffered weight losses following each immunization.
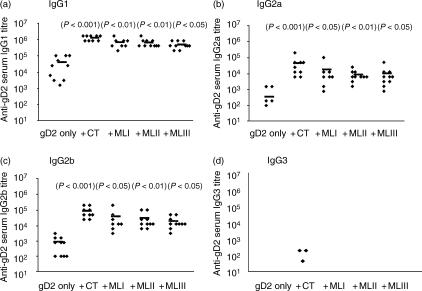
Analysis of the subclasses of gD2-specific IgG showed high titres of gD2-specific IgG1 in mice immunized with gD2 and lectins or CT (Fig. 2). Antigen-specific IgG2a and IgG2b were also detected in all mice but at a relatively lower titre. Significantly higher levels of specific antibody (IgG1, IgG2a and IgG2b) were detected in sera from mice immunized with gD2 and CT/mistletoe lectin than in mice immunized with the antigen alone. The ratio of gD2-specific IgG1 : IgG2a was approximately 27 : 1 (CT), 37 : 1 (MLI), 74 : 1 (MLII) and 47 : 1 (MLIII). When the CT/mistletoe lectin-specific IgG subclass titres were determined, an even more polarized response to the mistletoe lectins was observed. In this case, the IgG1 : IgG2a ratios were approximately 3 : 1 (CT), 337 : 1 (MLI), 216 : 1 (MLII) and 269 : 1 (MLIII) (Table 1). A higher specific IgG2b titre was generated when gD2 was co-administered with CT than with the mistletoe lectins (titres up to 20-fold lower in the case of the lectins). As with the gD2-specific responses, little CT/lectin-specific IgG3 was detected in any of the mice.
Figure 2.
Mistletoe lectins (ML) induce enhanced immunoglobulin G (IgG)1, IgG2a and IgG2b responses to nasotracheally co-administered glycoprotein D2 (gD2). Data show gD2-specific serum IgG subclass antibody titres in mice immunized on days 1, 14, 35 and 49 with either gD2 (5 µg) alone or gD2 (5 µg) together with cholera toxin (CT) (1 µg), MLI (1 µg), MLII (1 µg) or MLIII (1 µg). Titres of (a) IgG1, (b) IgG2a, (c) IgG2b and (d) IgG3 were measured 2 weeks after the final immunization. The diamond symbols represent antibody titres in individual animals and the symbol (–) represents the mean titre. P-values in parentheses refer to significance of data compared with the gD2-only group.
Mucosal IgA responses
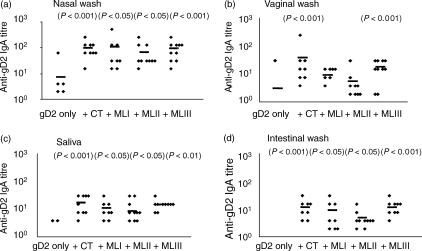
Co-administration of mistletoe lectins and gD2 induced gD2-specific IgA in all the mucosal secretions tested (Fig. 3). In the saliva, significantly higher levels of gD2-specific IgA were detected in mice receiving CT/mistletoe lectin and gD2 compared with the antigen alone. Similarly, in vaginal washes, levels of antigen-specific IgA were enhanced by co-administration of CT/lectins, although the titres were only significantly higher in the mice that received CT or MLIII with gD2. The stage of the menstrual cycle when the mice were killed was not assessed. This may partly explain the high degree of variability in the antigen-specific IgA titres. The highest titres of gD2-specific IgA were found in nasotracheal washes. Low levels of antibody were detected in washes from mice immunized with the antigen alone (five of 10) but higher titres were detected in the groups that received CT/mistletoe lectin with the antigen. Specific IgA was also detected in intestinal washes, a site distant from the point of immunization. In this case no response was detected in mice immunized with the antigen alone, while titres of up to 1 : 32 were detected in mice co-immunized with the antigen and CT/lectin. Comparing the three mistletoe lectins as adjuvants, the highest gD2-specific IgA titres in mucosal secretions were elicited by immunization with MLIII + gD2. Specific IgA responses were also induced by the lectins/CT when delivered by the intranasal route (Table 1). The highest titres were detected in mice immunized with CT; in this case relatively high, specific IgA titres were detected in all secretions, but particularly in nasotracheal washes. Mistletoe lectin-specific IgA was also elicited by immunization with MLI, MLII and MLIII at all the mucosal sites sampled. The highest titres were in nasotracheal washes, where the highest levels of lectin-specific IgA were induced by MLI.
Figure 3.
Mistletoe lectins (ML) enhance mucosal immunoglobulin A (IgA) responses to co-administered glycoprotein D2 (gD2) following nasotracheal immunization. Data show gD2-specific IgA antibody titres in secretions of mice immunized on days 1, 14, 35 and 49 with gD2 (5 µg) alone or gD2 (5 µg) together with cholera toxin (CT) (1 µg), MLI (1 µg), MLII (1 µg) or MLIII (1 µg). Responses were measured 2 weeks after the final immunization in (a) saliva, (b) vaginal wash, (c) nasotracheal wash and (d) intestinal wash. The diamond symbols represent antibody titres in individual animals and the symbol (–) represents the mean titre. P-values in parentheses refer to significance of data compared with the gD2-only group.
T-cell proliferation and cytokine responses
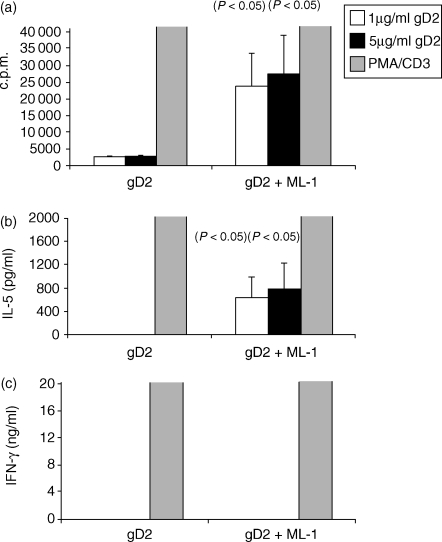
The objective of these experiments was to determine the ability of MLI to boost the immune responses to the bystander gD2 antigen. A potent proliferative response was detected when spleen cells from mice immunized with MLI and gD2 were restimulated in vitro with gD2 (Fig. 4a). This response was significantly higher than that in cells from mice immunized with the antigen alone. Delivery of gD2 alone did not induce the production of IL-5. However, IL-5 was produced by restimulated spleen cells from animals immunized with gD2 and ML-I (Fig. 4b). IFN-γ was not detected in supernatants from antigen-stimulated spleen cells, but was detected when cells were stimulated with PMA and anti-CD3. These data and the IgG subclass results indicate that ML1 induced a T helper 2 (Th2) type response to the co-administered gD2.
Figure 4.
Mistletoe lectin 1 (ML1) enhanced splenocyte antigen-specific proliferation and interleukin-5 (IL-5) production. IL-5 and interferon-γ (IFN-γ) were measured in supernatants by enzyme-linked immunosorbent assay (ELISA), and counts per minute (c.p.m.) (proliferation assay) by scintillation counting, 2 weeks after the last of three immunizations (days 0, 21, 42) with glycoprotein D2 (gD2) alone or gD2 together with ML1. Spleen cells were stimulated in vitro with medium only or with gD2 (1 µg/ml or 5 µg/ml) or phorbol 12-myristate 13-acetate (PMA) + anti-CD3. (a) proliferation; (b) IL-5 concentration in supernatants; and (c) IFN-γ concentration in supernatants. The key refers to the concentration of gD2 used to stimulate the cells or to the use of PMA + anti-CD3 as a positive control.
Discussion
The present study demonstrates that mistletoe lectins I, II and III are unique among the plant lectins tested to date in being strong mucosal adjuvants when co-administered with proteins via mucosal routes. To enhance the efficacy of a new generation of (generally) poorly immunogenic recombinant ‘subunit’ vaccines, there is a demand for adjuvants and delivery systems that are effective when administered by mucosal routes. To date, the most effective non-living mucosal adjuvants are the A-B toxins from Vibrio cholerae (CT) and Escherichia coli (LT) and their derivatives. Genetically detoxified mutants of these molecules are presently in clinical trials, while their non-toxic B (binding) subunits are being tested as vaccine carriers and immunomodulators.20
Mistletoe extracts have been extensively investigated in alternative cancer treatments and it has been proposed that the extracts, and specifically the lectin components, exert an immunomodulatory effect.21 Whether this mediates significant anti-cancer effects is the subject of much debate. RIPs have also been tested as components of immunotoxins for cancer therapy,5 and anti-human immunodeficiency virus (HIV) properties have been reported for some RIPs.22 Our work has revealed an additional application of these molecules as immunomodulators with potential in mucosal vaccine delivery. The three mistletoe lectins MLI, MLII and MLIII differ in their fine specificity of binding to cells.23 MLI exhibits a greater specificity for galactosyl residues, while MLIII has a higher affinity for N-acetyl galactosamine residues. MLII occupies an intermediate position in terms of its specificity for terminal sugars. Specific binding to and uptake by cells is an essential first step in eliciting an immune response after delivery via a mucosal route. This may facilitate enhanced receptor-mediated trans-epithelial uptake, possibly leading to entry into a non-proteolytic environment within the cell. The specific mechanism of uptake and intracellular processing of these lectins is under study.13
The present data indicate that mistletoe lectins induce a Th2 type response when co-administered with antigens by the nasotracheal route. In line with previous data,24 nasotracheally delivered CT elicited a Th2-type response to the co-administered antigen. CT and its B subunit (CTB) exert a number of effects on antigen-presenting cells, lymphocytes and epithelial cells.25–28
Our data on splenocyte cytokine responses (IL-5 production, no IFN-γ) and the high ratio of IgG1 : IgG2a indicate that MLI induced an even more polarized Th2 response to co-administered antigens than CT. The molecular basis for the induction of this type of response is at present unknown, but work is ongoing to assess the effects of the lectins on lymphocytes and antigen-presenting cells.
Mistletoe extracts used in ‘non-conventional’ cancer therapy contain a range of components, including MLI, MLII, MLIII, viscotoxins, membrane vesicles and polysaccharides.21 It is probable that MLI and its isoforms, MLII and MLIII, comprise the principal active agents in these preparations. Regular injections of standardized mistletoe extracts increased thymus weights and peripheral blood leucocyte counts in tumour-bearing mice.21 Furthermore, significant reductions in experimental liver and lung metastases were measured in mistletoe extract-treated mice.
Recently, the administration of mistletoe extracts to patients was shown to elicit a lectin-specific antibody response.29 Mistletoe extracts have been shown to increase the numbers30 and activity of granulocytes31 and to lead to an increase in leucocyte cell numbers.21 MLI bound specifically to human T-lymphoblastocytoid Jurkat cells via surface glycoprotein receptors, including the CD2 antigen, which is implicated in the induction of T-cell signalling events.32 Treatment of Jurkat cells with MLI [and specifically its B subunit (MLIB)] induced an increase in cytosolic calcium concentrations. MLIB also induced activation of the transcription factor NFAT. The lectin-induced calcium responses in Jurkat cells could be inhibited by CT and by inhibitors of protein kinases. This indicated that MLI could induce early T-cell activation events. The best-documented biological effect of mistletoe lectins is the induction of apoptosis; particularly in tumour cells lines and lymphocytes. MLIII was found to preferentially induce apoptosis of human CD8+ T cells with a memory phenotype (CD60Llo) in comparison with CD8+ CD60Lhi, CD4+ T cells or B cells.16 It was proposed that A-subunit-mediated inhibition of protein synthesis accelerates a receptor-mediated killing pathway induced by interaction of the B subunit with cell-surface receptors. This has parallels with the well-documented selective toxicity of CT and LT for CD8+ T cells.25
MLII has been shown to increase the phosphotransferase activity of c-Jun N-terminal kinase 1 (JNK1) in a variety of cell types, including T cells.33 We have preliminary evidence that mistletoe lectins can also modulate dendritic cells, resulting in the selective up-regulation of costimulatory molecules (E. C. Lavelle et al., unpublished). From the present and our previous2,3 data, it is clear that mistletoe lectins are strong mucosal adjuvants. Thus, in addition to evidence that mistletoe lectins are non-specific immunostimulants when applied clinically, our data show that the molecules are potent inducers of an antigen-specific immune response when administered mucosally. As detailed studies into the mucosal toxicity of the lectins have not been carried out in mice, it is not known if the lectins adversely affect the epithelium or mediate toxic effects systemically following uptake. Additionally, it is not known if there is a safety window within which the lectins could be used as mucosal adjuvants. Our preliminary data (unpublished) indicates that the lectins are considerably less toxic when administered via nasotracheal or intragastric routes than when injected. However, the most rational approach is to investigate the possibility that the enzyme activity of the lectins may be disassociated from their immunomodulatory activities, as is the case with LT. Our future work will focus on the generation of non-toxic mutants of mistletoe lectins and on their B subunits that are devoid of cytotoxic activities. To achieve this, the A- and B-chains of MLI have been cloned and sequenced.34–36
Acknowledgments
We wish to thank Rowett Research Services and SERAD for their support and assistance. The Chiron Corporation funded the study. The German Federal Ministry of Education and Research provided additional funding for the work on isolation and purification of mistletoe lectins.
References
- 1.Di Aizpurna HJD, Russell-Jones GL. Identification of classes of proteins that provide an immune response upon oral feeding. J Exp Med. 1988;167:440–51. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavelle EC, Grant G, Pusztai A, Pfüller U, O'Hagan DT. Mucosal immunogenicity of plant lectins in mice. Immunology. 2000;99:30–7. doi: 10.1046/j.1365-2567.2000.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavelle EC, Grant G, Pusztai A, Pfüller U, O'Hagan DT. The identification of plant lectins with mucosal adjuvant activity. Immunology. 2001;102:77–86. doi: 10.1046/j.1365-2567.2001.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peumans WJ, Hao Q, Van Damme EJ. Ribosome-inactivating proteins from plants: more than RNA N-glycosidases? FASEB J. 2001;15:1493–506. doi: 10.1096/fj.00-0751rev. [DOI] [PubMed] [Google Scholar]
- 5.Barbieri L, Battelli MG, Stirpe F. Ribosome-inactivating proteins from plants. Biochem Biophys Acta. 1993;1154:237–82. doi: 10.1016/0304-4157(93)90002-6. [DOI] [PubMed] [Google Scholar]
- 6.Barbieri L, Valbonesi P, Gorini P, Pession A, Stirpe F. Polynucleotide: adenosine glycosidase activity of saporin-L1: effect on DNA, RNA and poly (A) Biochem J. 1996;319:507–13. doi: 10.1042/bj3190507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh TA, Morgan AE, Hey TD. Characterization and molecular cloning of a proenzyme form of a ribosome-inactivating protein from maize. Novel mechanism of proenzyme activation by proteolytic removal of a 2.8-kilodalton internal peptide segment. J Biol Chem. 1991;266:23422–7. [PubMed] [Google Scholar]
- 8.Van Damme EJM, Hao Q, Charels D, Barre A, Rougé P, Van Leuven F. Characterization and molecular cloning of two different type 2 ribosome-inactivating proteins from the monocotyledonous plant Polygonatum multiflorum. Eur J Biochem. 2000;267:2746–9. doi: 10.1046/j.1432-1327.2000.01295.x. [DOI] [PubMed] [Google Scholar]
- 9.Peumans WJ, Van Damme EJM. Lectins as plant defense proteins. Plant Physiol. 1995;109:347–52. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsnes S, Stirpe F, Sandvig K, Phil A. Isolation and characterization of viscumin, a toxic lectin from Viscum album L. (mistletoe) J Biol Chem. 1982;257:13263–70. [PubMed] [Google Scholar]
- 11.Franz H. Mistletoe lectins and their A and B chains. Oncology. 1986;43:23–4. doi: 10.1159/000226417. [DOI] [PubMed] [Google Scholar]
- 12.Endo Y, Tsurugi K, Franz H. The site of action of the A-chain of mistletoe lectin I on eukaryotic ribosomes. The RNA N-glycosidase activity of the protein. FEBS Lett. 1988;231:378–80. doi: 10.1016/0014-5793(88)80853-6. [DOI] [PubMed] [Google Scholar]
- 13.Agapov II, Tonevitsky AG, Moysenovich MM, Maluchemko NV, Weyhenmeyer R, Kirpichnikov MP. Mistletoe lectin dissociates into catalytic and binding subunits before translocation across the membrane to the cytoplasm. FEBS Lett. 1999;452:211–4. doi: 10.1016/s0014-5793(99)00639-0. [DOI] [PubMed] [Google Scholar]
- 14.Pfüller U. Chemical constituents of european mistletoe (Viscum album L.). Isolation and characterisation of the main relevant ingredients: lectins, viscotoxins, oligo-/polysaccharides, flavonoides. In: Büssing A, editor. Mistletoe. The Genus Viscum. Amsterdam: Harwood Academic Publishers; 2000. pp. 101–22. [Google Scholar]
- 15.Ribéreau-Gayon G, Jung ML, Franz M, Anton R. Modulation of cytotoxicity and enhancement of cytokine release induced by Viscum album L. extracts or mistletoe lectins. Anti-Cancer Drugs. 1997;8:S3–S8. doi: 10.1097/00001813-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 16.Büssing A, Multani AS, Pathak S, Pfüller U, Schietzel M. Induction of apoptosis by the N-acetyl-galactosamine-specific toxic lectin from Viscum album L. is associated with a decrease of nuclear p53 and Bcl-2 proteins and induction of telomeric associations. Cancer Lett. 1998;130:57–68. doi: 10.1016/s0304-3835(98)00124-4. [DOI] [PubMed] [Google Scholar]
- 17.Jäggy C, Musielski H, Urech K, Schaller G. Quantitative determination of lectins in mistletoe preparations. Drug Res. 1995;45:905–9. [PubMed] [Google Scholar]
- 18.Kreitman RJ. Immunotoxins in cancer therapy. Curr Opin Immunol. 1999;11:570–8. doi: 10.1016/s0952-7915(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 19.Frankel AE, Kreitman RJ, Sausville EA. Targeted toxins. Clin Cancer Res. 2000;6:326–34. [PubMed] [Google Scholar]
- 20.Sun J-B, Mielcarek N, Lakew M, Grzych J-M, Capron A, Holmgren J, Czerkinsky C. Intranasal administration of a Shistosoma mansoni glutathione S-transferase-cholera toxoid conjugate vaccine evokes antiparasitic and antipathological immunity in mice. J Immunol. 1999;163:1045–52. [PubMed] [Google Scholar]
- 21.Braun JM, Ko HL, Schierholz JM, Weir D, Blackwell CC, Beuth J. Application of standardized mistletoe extracts augment immune response and down regulates metastatic organ colonization in murine models. Cancer Lett. 2001;170:25–31. doi: 10.1016/s0304-3835(01)00517-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang YX, Jacob J, Wingfield PT, et al. Anti-HIV and anti-tumor protein MAP30, a 30 kDa single-strand type-I RIP, shares similar secondary structure and beta-sheet topology with the A chain of ricin, a type-II RIP. Protein Sci. 2000;9:138–44. doi: 10.1110/ps.9.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bussing A, Stein GM, Pfuller U, Schnietzel M. Differential binding of toxic lectins from Viscum album L., MLI and MLIII, to human lymphocytes. Anticancer Res. 1999;19:5095–9. [PubMed] [Google Scholar]
- 24.Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and escherichia coli head-labile enterotoxins. Immunol Today. 1999;20:493–500. doi: 10.1016/s0167-5699(99)01523-6. [DOI] [PubMed] [Google Scholar]
- 25.Williams NA, Hirst TR, Nashar TO. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 26.Martin M, Metzger DJ, Michalek SM, Connell TD, Russell MW. Distinct cytokine regulation by cholera toxin and type II heat-labile toxins involves differential regulation of CD40 ligand on CD4(+) T cells. Infect Immun. 2001;69:4486–92. doi: 10.1128/IAI.69.7.4486-4492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagliardi MC, Sallusto F, Marinaro M, Langenkamp A, Lanzavecchia A, De Magistris MT. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur J Immunol. 2000;30:2394–403. doi: 10.1002/1521-4141(2000)30:8<2394::AID-IMMU2394>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 28.Bromander AK, Kjerrulf M, Holmgren J, Lycke N. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand J Immunol. 1993;37:452–8. doi: 10.1111/j.1365-3083.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 29.Klein R, Classen K, Berg PA, Ludtke R, Werner M, Huber R. In vivo induction of antibodies to mistletoe lectin-1 and viscotoxin by exposure to aqueous mistletoe extracts: a randomised double-blinded placebo controlled phase I study in healthy individuals. Eur J Med Res. 2002;7:155–63. [PubMed] [Google Scholar]
- 30.Hajto T, Hostanska K, Weber K, Zinke H, Fischer J, Mengs U, Lentzen H, Saller R. Effect of a recombinant lectin, Viscum album agglutinin on the secretion of interleukin-12 in cultured human peripheral blood mononuclear cells and on NK-cell-mediated cytotoxicity of rat splenocytes in vitro and in vivo. Nat Immun. 1998;16:34–46. doi: 10.1159/000069428. [DOI] [PubMed] [Google Scholar]
- 31.Braun JM, Gemmell CG, Beuth J, Ko HL, Pulverer G. Respiratory burst of human polymorphonuclear leukocytes in response to the galactoside-specific mistletoe lectin. Zentralbl Bakteriol. 1995;283:90–4. doi: 10.1016/s0934-8840(11)80894-7. [DOI] [PubMed] [Google Scholar]
- 32.Walzel H, Blach M, Neels P, Schulz U, Wollenhaupt K, Brock J. The B-chain of mistletoe lectin I efficiently stimulates calcium signalling in human Jurkat T-cells. Immunol Lett. 2001;78:57–66. doi: 10.1016/s0165-2478(01)00238-3. [DOI] [PubMed] [Google Scholar]
- 33.Park R, Kim M-S, So H-S, et al. Activation of c-Jun N-terminal kinase 1 (JNK1) in mistletoe lectin II-induced apoptosis of human myeloleukemic U937 cells. Biochem Pharmacol. 2000;60:1685–91. doi: 10.1016/s0006-2952(00)00482-2. [DOI] [PubMed] [Google Scholar]
- 34.Eck J, Langer M, Möckel B, Witthorn K, Zinke H, Lentzen H. Characterization of recombinant and plant-derived mistletoe lectin and their B-chains. Eur J Biochem. 1999;265:788–97. doi: 10.1046/j.1432-1327.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- 35.Eck J, Langer M, Möckel B, Baur A, Rothe M, Zinke H, Lentzen H. Cloning of the mistletoe lectin gene and characterization of the recombinant A-chain. Eur J Biochem. 1999;264:775–84. doi: 10.1046/j.1432-1327.1999.00638.x. [DOI] [PubMed] [Google Scholar]
- 36.Vervecken W, Kleff S, Pfüller U, Büssing A. Induction of apoptosis by mistletoe lectin I and its subunits. No evidence for cytotoxic effects caused by isolated A- and B-chains. Int J Biochem Cell Biol. 2000;32:317–26. doi: 10.1016/s1357-2725(99)00135-1. [DOI] [PubMed] [Google Scholar]