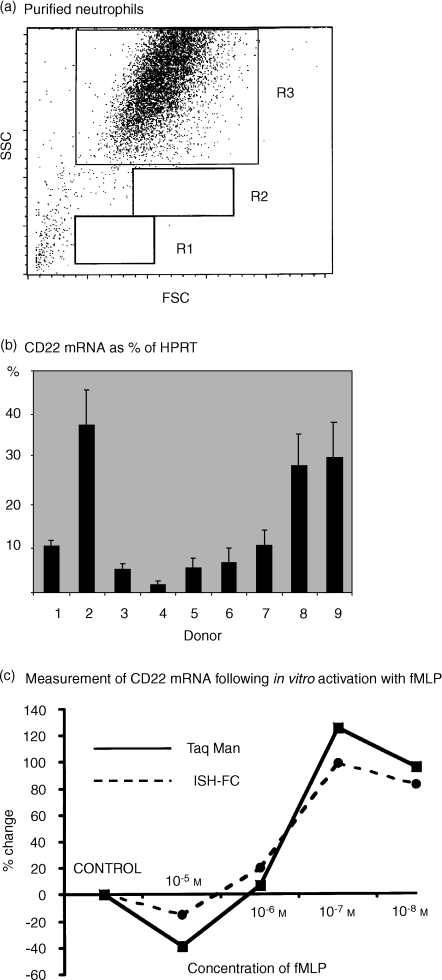
Figure 7.
Detection of mRNA specific for CD22 within normal and activated human peripheral blood neutrophils. Comparison of real-time PCR (TaqMan) and ISH-FC. (a) Dot-plots of forward versus side light scatter of human peripheral blood neutrophils isolated by the barrier method. Neutrophils gated as region 3 were all shown to express cell surface CD16, CD32 and CD35. Cells isolated by this method were virtually 100% pure as indicated by the lack of cells appearing in the lymphocyte (R1) and monocyte (R2) regions. (b) Using real-time PCR (TaqMan), mRNA for CD22 was detected to a variable extent in all of nine normal donors tested. Vertical bars indicate the mean ± SEM of triplicate measurements. Results are expressed as a percentage of a housekeeping gene (HPRT). (c) Highly purified human peripheral blood neutrophils were activated in vitro using various concentrations of fMLP. mRNA specific for CD22 was then detected in parallel using real-time PCR (TaqMan) and by ISH-FC). Results were expressed as the percentage change in mRNA relative to control cells which were incubated with culture medium alone.