Abstract
Bisphenol A (BPA) and p-nonylphenol (NP) are representative endocrine disruptors (EDs) that may have adverse effects on human health. The influence of these compounds on allergic immune responses remains unclear. In this study, we have examined the effects of BPA and NP on production of interleukin-4 (IL-4), a pro-inflammatory cytokine closely associated with allergic immune responses. Both BPA and NP significantly enhanced IL-4 production in keyhole limpet haemocyanin (KLH)-primed CD4+ T cells in a concentration-dependent manner. Treatment with BPA or NP in vivo resulted in significant increase of IL-4 production in CD4+ T cells and of antigen-specific immunoglobulin E (IgE) levels in the sera of KLH-primed mice. Furthermore, BPA and NP enhanced the activation of IL-4 gene promoter in EL4 T cells transiently transfected with IL-4 promoter/reporter constructs, and the enhancing effect mapped to a region in the IL-4 promoter containing binding sites for nuclear factor (NF)-AT. Activation of T lymphocytes by phorbol 12-myristate 13-acetate/ionomycin resulted in markedly enhanced binding activities to the NF-AT site, which significantly increased upon addition of BPA or NP, as demonstrated by the electrophoretic mobility shift assay, indicating that the transcription factor NF-AT was involved in the enhancing effect of BPA and NP on IL-4 production. The enhancement of IL-4 production by BPA or NP was significantly reduced by nitrendipine, a blocker of Ca2+ influx, and by FK506, a calcineurin inhibitor. FK506 inhibited the NF-AT–DNA binding activity and IL-4 gene promoter activity enhanced by BPA or NP. These results represent the first report describing possible enhancement of allergic response by EDs through increasing IL-4 production in CD4+ T cells and antigen-specific IgE levels in the sera via the stimulation of Ca2+/calcineurin-dependent NF-AT activation.
Introduction
Exogenous substances that can elicit sex steroid-like activities are commonly referred to as endocrine disruptors (EDs). They have been defined as any exogenous agent, either synthetic or natural, that interferes with the production, release, transport, metabolism, binding, biologic action, or elimination of natural ligands in the body that are responsible for the maintenance of homeostasis and the regulation of developmental processes. In many cases, EDs share no apparent structural similarities to traditional steroids. The potential exposure and economic significance of several of these substances have made endocrine-disrupting chemicals a contentious health concern and environmental issue.1,2 In humans, the consequences of prenatal exposure to diethylstilbesterol on the reproductive tract of both females and males are well known. Developmentally neurological defects have been identified in children exposed to EDs.3,4 In addition, a decline of sperm production in humans over the last four decades has been noted. Increases in the incidence of certain types of cancers (breast, prostate, testicular) that may have an endocrine-related basis have led to speculation that they may be caused by agents in the environment.5 Environmental exposures to EDs are also suspected to play a role in alterations of sexual development in wildlife species.
The effect of EDs on cytokine production or the function of the immune system has not been investigated. They may have an adverse effect. We chose two widely used EDs for our study: bisphenol A (BPA), which is found in the content of canned food, dental sealants, and composites; and nonylphenol (NP), alkylphenols used as antioxidants in the plastic industry.6,7 BPA is used in the manufacture of epoxy, polycarbonate and unsaturated polyester resins. It is also used in the manufacture of epoxy di(meth)acrylates and vinyl ester resins, and has been used as an antioxidant, fungicide, and antimicrobial in cosmetics.8 Diglycidyl ether of BPA epoxy resins belong to the most common causes of occupational allergic contact dermatitis and, on rare occasions, has caused occupational asthma.9 Also, the non-ionic emulsifier nonylphenol ethoxylate (nonoxynol-6) found in an industrial waterless hand cleanser induced allergic contact dermatitis.10 However, the mechanism by which EDs causes these allergic responses is unknown. In this study, we determined if allergic responses induced by EDs were associated with an increase in interleukin-4 (IL-4) production by T cells and/or an increase in serum immunoglobulin E (IgE) level.
Allergic disorders affect at least 20% of the population in developed countries. They include hay fever, asthma, atopic dermatitis and food allergies. These signs are associated with high levels of serum IgE and allergen-specific IgE and eosinophilia.11,12 They are dependent upon IL-4 and IL-5 released from allergen-specific CD4 T cells expressing the T helper type 2 (Th2) cytokine profile.13,14 IL-4 is a pleiotropic cytokine that modulates the differentiation and the biological activities of virtually all cells of haematopoietic origin.15 It plays a central role in Th2-type immune responses, such as IgE production and immediate allergic inflammation. It may be involved in the exacerbation of allergic diseases.16
The objective of this study was to determine the effect of EDs on IL-4 production in antigen-primed lymph node cells. We found that BPA and NP, two widely used EDs, up-regulated keyhole limpet haemocyanin (KLH)-induced IL-4 production in lymph node cells of antigen-primed mice, and that this effect was mediated in part by nuclear factor (NF)-AT sites in the murine IL-4 promoter. Because the Ca2+ signalling system was involved in IL-4 production, we investigated the role of these intracellular signalling systems and found that the enhancing effect of BPA and NP on IL-4 production was antagonized by interruption of intracellular Ca2+ signalling with 1,2-bis(o-amionophenoxy)ethane-N,N,N ′,N ′-tetraacetic acid tetra(acetoxymethyl)ester (BAPTA-AM), thapsigargin, nitrendipine and FK506.
Materials and methods
Materials, cell culture and mice
BPA and NP were from Aldrich Chemical Co. (Milwaukee, WI) and Tokyo Kasei Kogyo Co. (Tokyo, Japan), respectively. Phorbol 12-myristate 13-acetate (PMA), ionomycin, and antagonists of the Ca2+ signalling systems were from Sigma Chemical Co. (St. Louis, MO). KLH was from Calbiochem Co. (San Diego, CA). Anti-CD8 monoclonal antibody (mAb; Lyt-2.2, hybridoma 3.155) and anti-CD4 mAb (L3T4, hybridoma GK1.5) were purified from ascitic fluids by ammonium sulphate precipitation. Hybridoma 3.155 cells, hybridoma GK1.5 cells and EL4 cells were from the American Type Culture Collection (ATCC, Rockville, MD). Anti-mIL-4 (BVD4 and BVD6), anti-mouse-interferon-γ (IFN-γ) (R46A2 and XMG1.2), anti-mouse IgE, purified mouse IgE, biotinylated anti-mouse IgE, recombinant murine IL-4 and IFN-γ were from PharMingen Co. (San Diego, CA). Cultures of lymph node cells from BALB/c mice were maintained in RPMI-1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT) and 1% penicillin–streptomycin at 37° in a 5% CO2 humidified air atmosphere. Six to 8-week-old-female BALB/c mice were obtained from Daehan Animal Inc. (Seoul, Korea), and maintained in pathogen-limited conditions. The mice were treated according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
In vitro stimulation of lymph node cells
Draining axillary, popliteal, and inguinal lymph nodes were removed from mice 7 days after priming with 100 µg KLH absorbed to aluminium hydroxide (alum) adjuvant in the footpads as previously described.17 Single-cell suspensions of lymph nodes were prepared and cultured in vitro with KLH (1 or 50 µg/ml) in the absence or presence of either BPA or NP. At the indicated times as described in the figure legends, the levels of IL-4 and IFN-γ in the cell supernatants were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) and mRNA levels of IL-4, IL-6 and IL-10 in the cells were assayed by reverse transcription–polymerase chain reaction (RT–PCR).
In vitro depletion of T-cell subsets
For in vitro depletion of either CD8+ T cells or CD4+ T cells, mAbs for the relevant T-cell subset were used as previously described.18 In brief, lymph node cells from immunized mice were incubated with anti-CD4 (L3T4) or anti-CD8 (Lyt-2.2) mAbs on ice for 30 min, followed by addition of low-toxicity rabbit complement (Pel-Freez, Rogers, AR) at 37° for 45 min. The antibodies were titred such that the concentrations used were five times the minimum amount required to saturate the specific binding sites of lymph node cells from naive BALB/c mice, as determined by cytofluorometric analysis of serially diluted antibodies. After depletion of the specific cell-types, the remaining cells were washed with serum-free RPMI-1640 medium, and incubated for 4 days with KLH (50 µg/ml) in the presence of BPA (10 or 50 µm) or NP (1 or 5 µm). Immunofluorescent analysis of lymph node cells after mAb treatment indicated that there were > 95% depletion of specific T-cell subsets with no decrease in the frequency of the other subsets.
Immunizations, in vivo treatments, and in vitro culture
Mice (five mice/group) were injected into the footpad with KLH (100 µg) in alum, followed by intraperitoneal (i.p) injection every other day for 1 week with BPA (25 mg/kg), NP (5 mg/kg) or saline. Unimmunized, control mice were footpad injected with saline, followed by i.p. injection with saline. One week after the second injection with KLH, lymph nodes and blood samples were collected. Serum samples were rapidly frozen at − 80° for the subsequent determination of KLH-specific IgE levels. The lymph node cells were stimulated in vitro with KLH (0–100 µg/ml) for 4 days and the levels of IL-4 and IFN-γ in the culture supernatants were determined by a sandwich ELISA.
Cytokine assays
The quantities of IL-4 and IFN-γ in culture supernatants were determined by a sandwich ELISA using mAbs specific for each cytokine, as previously described.19 The mAbs for coating the plates and the biotinylated second mAbs were as follows: for IL-4, BVD4-1D11 and BVD6; for IFN-γ, HB170 and XMG1.2. Standard curves were generated using recombinant cytokines. The lower limit of detection was 3 pg/ml for IL-4, and 125 pg/ml for IFN-γ.
IgE assay
Mice were bled 1 week after immunization and KLH-specific IgE levels in the sera were determined by ELISA, using rat anti-mouse IgE (1 µg/ml) to coat the plates. After the samples were applied, the plates were washed and biotinylated KLH (1 µg/ml) was added. The plates were washed and developed with streptavidin-peroxidase in phosphate-buffered saline (PBS).
RT–PCR
Total RNA was prepared from the cells and reverse-transcribed into cDNA, and then PCR amplification of the cDNA was preformed as previously described.20 Total cellular RNA was isolated by the single-step method using the TRI reagent (Sigma). The sequences of PCR primers were as follows: mouse IL-4 (sense, 5′ATGGGTCTCAACCCCCAGCTAGT3′; antisense, 5′GCTCTTTACGCTTTCCAGGAAGTC3′), IL-6 (sense, 5′TGAACAACGATGATGCACTT3′; antisense, 5′CGTAGAGAACAACATAAGTC3′), IL-10 (sense, 5′ATGCAGGACTTTAAGGGTTACTTGGGT3′; antisense, 5′ATTTCGGAGAGAGGTACAAACGAGGTTT3′), and β-actin (sense, 5′TGGAATCCTGTGGCATCCATGAAAC3′; antisense, 5′TAAAACGCAGCTCAGTAACAGTCCG3′). The PCR reactions were run for 36 cycles for 94° (30 s), 58° (45 s), 72° (30 s) using a PCR Thermal Cycler (MJ Research, Watertown, MA). After the amplification, the RT–PCR products were separated in 1·5% (w/v) agarose gels and stained with ethidium bromide.
IL-4 promoter constructs, transient transfection and luciferase assay
The 741/+56 fragment of murine IL-4 promoter was generated by polymerase chain reaction (PCR) from genomic DNA of DBA/2 mice. The PCR products were cloned into the BamHI/EcoRI sites of the plasmid pGEM-7Z and then subcloned into the SacI/XhoI sites of the pGL3-basic luciferase vector (Promega Co., Madison, WI). All the deletion mutants were generated by PCR using an upstream primer containing BamHI site. For transfections, EL4, a murine thymoma cell line, was cultured in RPMI-1640 medium and transfected with indicated plasmid in the presence of Superfectam according to the manufacturer's protocol (Qiagen, Germany). The cells were stimulated with ionomycin (100 nm) and PMA (1 ng/ml) in the absence or presence of varying concentrations of either BPA or NP. The cells were harvested 24 hr later, and the luciferase activity was assayed. The results were normalized to LacZ expression and expressed as a relative fold induction.
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
EL4 cells were stimulated by the addition of PMA (1 ng/ml) plus ionomycin (100 nm), and incubated for 24 hr with BPA or NP at the indicated amounts. Nuclear extracts were prepared from the cells as previously described,21 and aliquots were frozen at −80°. The protein concentration for each extract was determined with a Micro BCA protein assay reagent (Pierce, Rockford, IL). For binding assays, 10 µg of total nuclear extract was incubated with 32P-labelled oligonucleotide in the presence of a reaction mixture containing 20 mm dithiothreitol, poly(dI-dC), 10× gel retardation assay buffer (GRAB) for 30 min at room temperature. The oligonucleotides used in binding and competition assays were as follows: NF-AT, 5′-GAGCCCTAAACTCATTTTCCCTTGAAA-3′; AP-1, 5′-GATCTGCATGAGTCAGACACACA-3′; NF-κB, 5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′; CRE, 5′-GATCCGAGCCCGTGACGTTTACACTCATTCT-3′. For the competition assay, a 50-fold excess amount of the appropriate unlabelled oligonucleotide was added to the binding reaction mixture. The binding reactions were loaded onto a 4% polyacrylamide gel in 0·5× Tris–borate–ethylenediaminetetra-acetic acid buffer and subjected to electrophoresis at 200–250 V for 1 hr. Dried gels were exposed to an X-ray film at −80°.
Statistical analyses
Student's t-test and one-way analysis of variance (anova) were used to determine the statistical differences between values for various experimental and control groups.
P-values < 0·01 were considered statistically significant.
Results
BPA and NP enhanced IL-4 production by KLH-primed lymph node cells and PMA-stimulated EL4 T cells
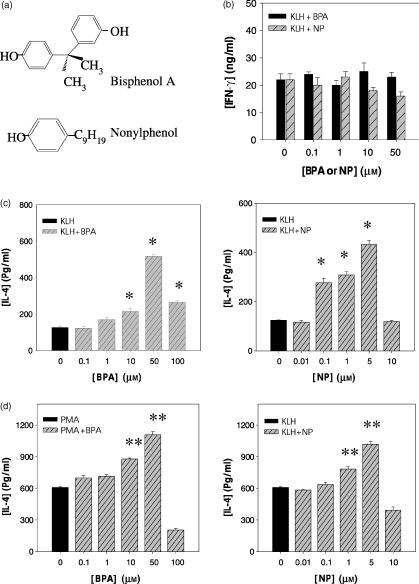
To determine whether BPA and NP affected the production of IL-4 by lymph node cells primed with KLH, BALB/c mice were first injected into the footpad with KLH (100 µg) in alum. Seven days later, lymph node cells from the immunized mice were stimulated for 4 days in vitro with KLH in the presence of BPA or NP, and IL-4 production by KLH-specific cells was determined. As shown in Fig. 1, BPA and NP significantly increased IL-4 production in a concentration-dependent manner, with the highest levels of IL-4 at 50 and 5 µm, respectively. BPA and NP also stimulated IL-4 production in PMA-activated EL4 T-cell line. BPA or NP itself did not induce IL-4 production by unstimulated lymph node cells or EL4 T cells. In contrast, BPA did not affect the production of IFN-γ, a Th1 cytokine, in KLH-stimulated lymph node cells.
Figure 1.
Chemical structures of BPA and NP, and their effects on IL-4 production in KLH-primed lymph node cells and PMA-activated EL4 cells. (a) Chemical structures of test chemicals: bisphenol A and 4-nonylphenol. (b, c) Mice were injected into the footpad with KLH (100 µg) in alum. Seven days later, the lymph node cells were collected and stimulated in vitro for 4 days with KLH (50 µg/ml) in the presence of varying amounts of BPA or NP. (d) EL4 thymoma cells were treated with PMA (1 ng/ml), and then exposed to varying amounts of BPA or NP for 2 days. The cell culture supernatants were harvested and assayed for IL-4 or IFN-γ by ELISA. The values represent the mean ± SEM (n = 4). *P < 0·001 and **P < 0·005 relative to KLH-treated and PMA-treated group, respectively.
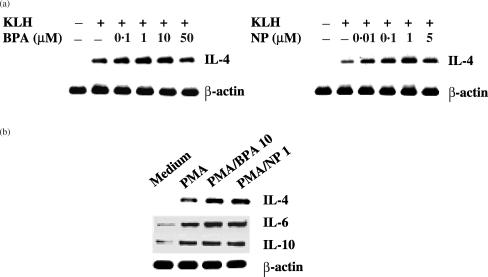
IL-4 mRNA levels were analysed in KLH-primed lymph node cells in the presence of BPA or NP, to determine if changes in IL-4 production were accompanied by changes in the expression of IL-4 mRNA. As shown in Fig. 2, BPA and NP significantly enhanced IL-4 mRNA levels in both KLH-primed lymph node cells and PMA-activated EL4 T cells, indicating that the changes in IL-4 production with BPA or NP were present at the transcriptional level. The enhancing effect of BPA and NP on IL-4 mRNA expression was approximately maximal at 10 and 1 µm, respectively, and decreased at higher concentrations. Treatment with BPA or NP did not affect the expression of IL-6, IL-10 and β-actin mRNA by PMA-activated EL4 T cells, suggesting that the enhancing effect of IL-4 by BPA or NP was not the result of generalized activation of these cells.
Figure 2.
Effect of BPA or NP on IL-4 mRNA expression by KLH-stimulated lymph node cells and PMA-activated EL4 cells (a) Lymph node cells from KLH-primed mice, as described in the legend to Fig. 1(b), were re-stimulated for 6 hr with KLH (1 µg/ml) in the presence of varying amounts of BPA or NP. (b) EL4 cells were stimulated with PMA (1 ng/ml), and then exposed to BPA (10 µm) or NP (1 µm) for 6 hr. Cellular RNA from each treatment was extracted and mRNA expression for IL-4, IL-6, IL-10 and β-actin was analysed by RT–PCR.
CD4+ T cells were the major cell-type activated for IL-4 by BPA or NP
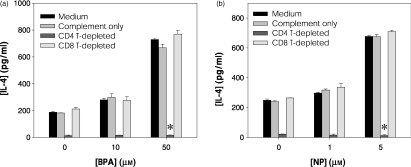
IL-4 is produced predominantly by CD4+ Th2 cells and CD8+ T cells.22 To determine the IL-4-producing cell type affected by treatment with BPA or NP, CD4+ or CD8+ T cells in KLH-primed lymph node cells were depleted with anti-CD4 or anti-CD8 mAbs plus complement. The depleted cell cultures were then stimulated in vitro with KLH in the presence of BPA or NP. Afterwards, IL-4 levels in the depleted cell cultures were compared with those of nondepleted cell cultures after in vitro stimulation with KLH. As shown in Fig. 3, in vitro re-stimulation of KLH-primed lymph node cells with KLH induced significant levels of IL-4 production, which was reduced to background levels by CD4+ T-cell depletion. In contrast, CD8+ T-cell depletion did not affect the level of IL-4 in cultures of KLH-primed lymph node cells in the presence of BPA or NP. Thus, CD4+ T cells were the major producer of IL-4 in cultures of KLH-activated lymph node cells.
Figure 3.
CD4+ T cells are the major cell-type responsive to BPA or NP for IL-4 production. Mice were injected in the footpad with KLH in alum. Seven days later, draining lymph node cells were incubated in vitro on ice for 30 min with anti-CD8 or anti-CD4 mAbs, followed by incubation for 45 min at 37° with complement. After washing, cells from each treatment group were stimulated for 4 days with KLH (50 µg/ml) in the presence of the indicated doses of BPA (a) or NP (b) and IL-4 levels in the culture supernatants were assayed by ELISA. The data represent the mean ± SEM (n = 3). *P < 0·0005 relative to a group treated with complement only.
As described, BPA or NP significantly increased IL-4 production by KLH-primed lymph node cells. The enhanced levels of IL-4 production by BPA or NP were also completely suppressed by CD4+ T-cell depletion. IL-4 levels in CD4+ T-cell depleted lymph node cells in the presence of KLH and BPA or NP were approximately the same as background (unstimulated control cell culture), indicating that CD4+ T cells were the major cell-type responsible for IL-4 production in KLH-primed lymph node cells, and for the enhanced production of IL-4 by treatment with BPA or NP.
BPA and NP increased allergic responses in vivo, characterized by enhanced IL-4 production in CD4+ T cells and elevated antigen-specific IgE in sera
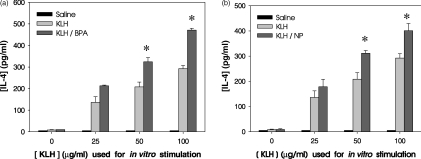
To determine whether BPA or NP up-regulated IL-4 levels in vivo, BPA or NP was injected i.p (25 and 5 mg/kg every other day, respectively, during the KLH immunization) into KLH-primed BALB/c mice. One week after the KLH injection, lymph node cells were collected and stimulated in vitro with KLH, and the level of IL-4 in the culture supernatants was determined. As shown in Fig. 4, treatment with BPA or NP significantly increased IL-4 production in lymph node cells of KLH-primed mice after in vitro stimulation with KLH. The levels of IL-4 production in KLH/BPA- or KLH/NP-treated mice were significantly higher than those in mice injected with KLH alone.
Figure 4.
IL-4 production by lymph node cells in KLH-primed mice treated in vivo with BPA or NP.Mice (5 per group) were injected in the footpad with 100 µg KLH in alum. The mice were injected i.p. with BPA (25 mg/kg) (a), NP (5 mg/kg) (b) or saline every other day for 1 week. Control mice received saline alone. One week after the second immunization with KLH, lymph node cells were re-stimulated in vitro with KLH (0–100 µg/ml) for 4 days. IL-4 levels in the culture supernatants were analyzed by ELISA. The data represent the mean ± SEM (n = 3). *P < 0·01 relative to groups without BPA or NP treatment.
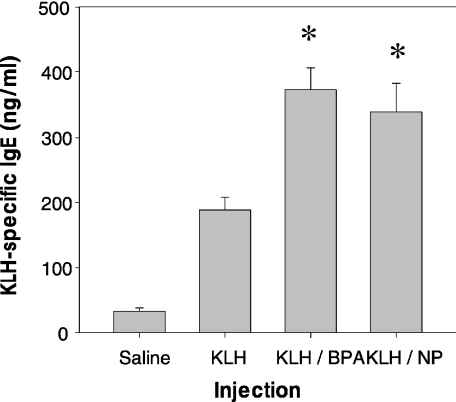
To further characterize allergic responses induced with BPA or NP, serum levels of KLH-specific IgE in KLH-primed mice treated with BPA or NP were determined. As shown in Fig. 5, the levels of KLH-specific IgE in BPA- or NP-treated mice were significantly higher than those in mice injected with KLH alone. The results suggest that BPA or NP may enhance allergic immune responses in antigen-primed mice by increasing IL-4 production in CD4+ T cells and the level of antigen-specific IgE in the serum.
Figure 5.
Effect of BPA or NP on KLH-specific IgE levels in the sera of KLH-primed mice. Mice (five per group) were treated with the same protocol as described in the legend of Fig. 4. One week after the second immunization with KLH, KLH-specific IgE levels in the sera were determined by ELISA. The experiment was repeated twice with similar results. *P < 0·01 relative to a group treated with KLH alone.
BPA and NP enhanced the activation of the IL-4 gene promoter by PMA/ionomycin
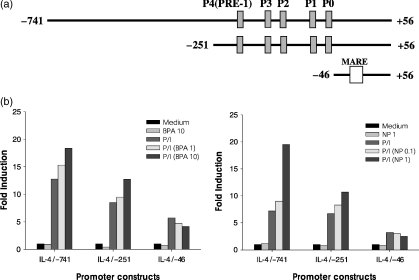
To determine if BPA or NP affected the activity of the IL-4 gene promoter, luciferase reporter constructs were generated that contained the IL-4 promoter sequences from positions −741, −251, and −46 to +56, relative to the transcription initiation site (Fig. 6). EL4 T cells were transfected with each of the IL-4 gene promoter constructs and stimulated with PMA/ionomycin in the presence of varying concentrations of BPA or NP. Afterward, the luciferase activity was determined. As shown in Fig. 6, each construct showed significant stimulation with PMA/ionomycin in the absence of BPA or NP. Both BPA and NP significantly enhanced PMA/ionomycin-mediated activation of the IL-4 gene promoter. Of special interest, deleting sequences to −46 (IL-4/−46) removed the enhancing effects of BPA or NP on the activation of the IL-4 promoter, suggesting that the target site of BPA or NP may residue at the region carrying four NF-AT sites (Pu-bD, Pu-bC, Pu-bB and Pu-bA). BPA or NP alone did not stimulate the IL-4 gene promoter.
Figure 6.
Effect of BPA or NP on IL-4 gene promoter activity stimulated by PMA/ionomycin. (a) Schematic representation of murine IL-4 promoter constructs containing P0–P4 NF-AT elements. (b) EL4 cells were transiently transfected with the IL-4 promoter constructs, followed by stimulation for 24 hr with PMA/ionomycin in the presence of BPA or NP. Afterward, the cells were lysed and reporter gene expression was analysed by luminometry. The results are represented as induction fold over the value obtained with the unstimulated EL4 cells transfected with each of promoter constructs, given as an arbitrary value of 1. The data are representative of three independent experiments.
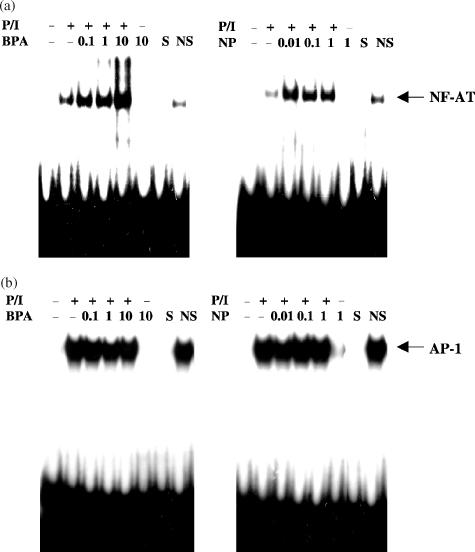
To further examine the involvement of NF-AT in the enhancement of IL-4 production by BPA or NP, we analysed the NF-AT-binding activity present in nuclear extracts derived from PMA/ionomycin-stimulated EL4 T cells in the presence of BPA or NP. As expected, nuclear extracts from PMA/ionomycin-stimulated EL4 cells exhibited strong NF-AT-binding activity as determined by electrophoretic mobility shift assays using radiolabelled oligonucleotides encompassing the NF-AT site (Fig. 7a). The binding was specific since it was competed by an unlabelled, identical oligonucleotide, but not with an unrelated non-specific oligonucleotide. It was absent in nuclear extracts from non-stimulated cells. Nuclear extracts from EL4 cells stimulated by PMA/ionomycin in the presence of BPA or NP showed strong NF-AT-binding activities, in a dose-dependent manner. In contrast, BPA or NP failed to enhance the AP-1 binding activity induced by PMA/ionomycin (Fig. 7b).
Figure 7.
BPA or NP enhances the PMA/ionomycin-induced binding of nuclear proteins to the NF-AT site, but not the AP-1 site. Nuclear extracts were prepared from resting and PMA/ionomycin-activated EL4 cells treated for 24 hr with varying concentrations of BPA or NP, and then analysed by EMSA using a radiolabelled oligonucleotide encompassing the NF-AT (a) or AP-1 (b), respectively. S and NS indicate the presence of an unlabelled, specific oligonucleotide (NF-AT or AP-1) and non-specific oligonucleotide (CRE or NF-κB), respectively. The specific NF-AT or AP-1 complexes are indicated. Similar results were obtained in two additional experiments.
Involvement of Ca2+ in the BPA- or NP-stimulated production of IL-4
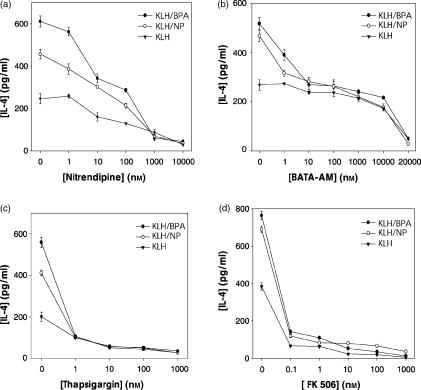
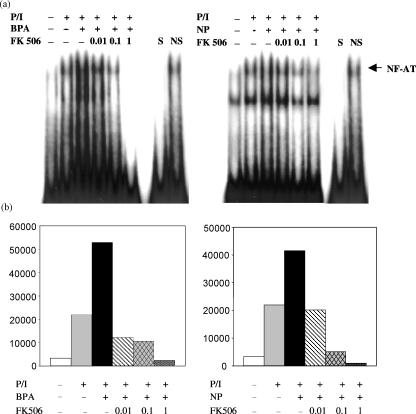
We assessed the contribution of intracellular calcium-mediated signalling to BPA- or NP-enhanced IL-4 production by pretreating lymph node cells from the immunized mice with various inhibitors known to interfere with calcium homeostasis. Both BPA and NP increase intracellular [Ca2+] levels by inhibiting intracellular Ca2+ pumps.23 As shown in Fig. 8 (a and b), the enhancement of IL-4 production by BPA or NP was significantly reduced by nitrendipine, which blocks Ca2+ influx, or BAPTA-AM, a chelator of intracellular Ca2+. In agreement with the data on chelation of intracellular calcium by BAPTA-AM (Fig. 8b), thapsigargin, which used to inhibit calcium-dependent ATPases located in the sarco/endoplasmic reticulum, which release calcium from intracellular stores, completely suppressed IL-4 production enhanced by BPA or NP (Fig. 8c). We further determined whether Ca2+/calmodulin downstream enzyme was involved in IL-4 production stimulated by BPA or NP. As shown in Fig. 8(d), when KLH-primed lymph node cells were pretreated with FK506, a calcineurin inhibitor, the enhancement of IL-4 production by BPA or NP was completely abolished. Furthermore, FK506 inhibited the NF-AT-DNA binding activity and IL-4 gene promoter activity enhanced by BPA or NP, in a concentration-dependent manner (Fig. 9).
Figure 8.
The role of Ca2+/calcineurin-dependent intracellular signalling systems in the enhanced IL-4 production by BPA or NP. Mice were injected in the footpad with KLH in alum. Seven days later, lymph node cells were incubated with nitrendipine (a), BAPTA-AM, (b), thapsigargin (c) and FK506 (d) 30 min prior to KLH and/or BPA or NP stimulation. After 4 days, the cell culture supernatants were harvested and assayed for IL-4 by ELISA. The values represent the mean ± SD of triplicate determinations. The experiment was repeated twice with similar results.
Figure 9.
Effect of FK506, a calcineurin inhibitor, on the NF-AT binding activity and IL-4 gene promoter activity. (a) EL4 cells were pretreated for 30 min with FK506 (10, 100, 1000 nm) and then exposed for 24 hr to PMA/ionomycin (P/I) in the presence of BPA (10 µm) or NP (1 µm). In EMSA a 32P-end-labelled DNA probe that included the NF-AT binding domain of the murine IL-4 promoter was incubated with nuclear extracts (10 µg/lane). Specificity of binding was determined by the use of a 50-fold excess of specific or unrelated oligonucleotide (CRE). (b) EL4 cells were transiently transfected with the IL-4/−741/Luc construct. Eighteen hr later, the cells were pretreated for 30 min with FK506 (10, 100, 1000 nm). The results are the averages of three independent transfection experiments.
Discussion
In this report, we demonstrated for the first time that BPA and NP, two representative EDs, significantly enhanced IL-4 production by antigen-primed CD4+ T cells. In agreement with the increased IL-4 production observed in vitro, mice treated with BPA or NP during sensitization produced higher levels of IgE in vivo, and lymph node cells derived from these mice secreted enhanced amounts of IL-4 following antigen stimulation in vitro. Furthermore, we found that the enhancement of IL-4 by BPA or NP was mediated by a Ca2+/calcineurin/NF-AT signalling pathway. These results suggested that BPA and NP may, at least in part, increase allergic responses via the enhancement of IL-4 production by CD4+ T cells.
Allergic diseases are hypersensitivity disorders associated with the production of specific IgE to environmental allergens.24 Higher than normal serum IgE is often found in patients with allergic diseases, including allergic asthma. Reduction of IgE production is one strategy used in the treatment of asthma.25,26 IL-4, produced primarily by CD4+ Th2 cells, is an important stimulus for the switch of the antibody isotype to IgE in both mice and humans.27,28 In this study, the increased levels of IgE in sera of BPA- or NP-treated mice may have resulted from an enhancement in the production of IL-4 by CD4+ T cells exposed to BPA or NP, leading to the enhancement of the allergic response.
BPA and NP were found to significantly enhance IL-4 mRNA levels in a concentration- and time-dependent manner (Fig. 2). It also enhanced the activation of the IL-4 gene promoter by PMA/ionomycin (Fig. 6), indicating that the enhancement of IL-4 production by BPA or NP occurred at the transcriptional level. Furthermore, BPA or NP enhanced signals originating from the T-cell receptor, leading to a higher expression of IL-4 during antigen stimulation. BPA and NP may also directly interact with signal pathways or transcription factors regulating the IL-4 gene promoter, resulting in an increase in the expression of IL-4. In our study, the enhancing effect of BPA or NP on the PMA/ionomycin-activated IL-4 gene promoter disappeared if the transcription factor NF-AT sites were deleted in the promoter (Fig. 6), suggesting that the enhancing effect of BPA or NP on IL-4 production may be mediated through NF-AT. Furthermore, activation of T cells by PMA/ionomycin resulted in a marked enhanced binding activity at NF-AT sites, which significantly increased upon addition of BPA or NP. The transcription factor NF-AT is well known to play an essential role in the inducible transcription of the IL-4 gene during T-cell activation, as human and murine IL-4 gene promoters contain at least four NF-AT sites, which control their induction in T cells.29,30 AP-1 is required for the activity of NF-AT DNA binding to the NF-AT sites.31 However, our study showed that the AP-1 DNA binding activity was not modulated by the treatment with BPA or NP, indicating that AP-1 may not be involved in the enhancing effects of BPA or NP on IL-4 production by CD4+ T cells.
NF-AT-dependent enhancement of IL-4 production by BPA and NP might occur through the Ca2+/calcineurin signalling pathway. The enhancement of IL-4 production by BPA or NP was completely reduced by nitrendipine, a blocker of Ca2+ influx, or BAPTA-AM, a chelator of intracellular Ca2+ (Fig. 8). Furthermore, enhancement of IL-4 production by BPA or NP was completely abolished by FK506, a calcineurin inhibitor. Treatment with FK506 inhibited the NF-AT–DNA binding activity and IL-4 gene promoter activity enhanced by BPA or NP, in a dose-dependent manner (Fig. 9), suggesting that BPA and NP enhanced IL-4 production via a Ca2+/calcineurin signalling pathway, resulting in the elevated activation of NF-AT–DNA binding activity. The role of calcium influx in cytokine generation has long been recognized, and mounting evidence linked the cytosolic increases of Ca2+ to intracellular signals involving the activation of calcineurin A (a serine-threonine phosphatase) and its effect on the NF-AT family of transcription factors.32,33 The transmission of signals derived from T-cell stimulation leads to the activation of the Ca2+/calcineurin pathway, which in turn triggers the translocation of NF-AT to the nucleus. Proteins belonging to the NF-AT family of transcription factors control the transcription of many genes involved in allergy and eosinophil function.34 NF-AT proteins are expressed in T cells, B cells, mast cells, and natural killer cells, where they are activated by stimulation of calcium-mobilizing antigen and Fc receptors. They regulate the expression of appropriate inducible genes.35,36 Upon calcium signalling, the Ca2+/calmodulin-dependent protein phosphatase calcineurin dephosphorylates NF-AT proteins lead to the unmasking of the nuclear localization sequences and the translocation of NF-ATs to the nucleus. NF-ATs remain in the nucleus while Ca2+ is elevated and are rapidly phosphorylated and exported to the cytoplasm upon termination of calcium signalling or by calcineurin inhibition with cyclosporin A or FK506.37,38
The amounts of BPA or NP used in this study (25 mg/kg BPA and 5 mg/kg NP) seem high, especially in the in vivo experiments. However, many previous reports have used similar amounts of BPA (0·1–150 mg/kg body weight) to investigate the effect of BPA on gene expression and bioavailability because the obtained serum concentration following BPA administration was low and the systemic clearance was high and the elimination half-life was short. For example, the lowest BPA dose that had a significant effect on vascular endothelial growth factor expression was 37·5 mg/kg after i.p. administration.39 Upon oral administration of BPA (10 mg/kg), the maximum serum concentration and the time to reach the maximum concentration were 14·7 ng/ml and 0·2 hr, respectively.40 The relative bioavailability and metabolism were also dependent upon the route of administration.41
Epidemiological studies indicated an increase in the prevalence of allergic diseases in environmentally polluted areas, suggesting that environmental factors may be one of the causes of the disease.42 Recently, several environmental pollutants have been reported to increase allergic responses. Pyrene, one of the polyaromatic hydrocarbons in diesel exhaust, induced transcription of IL-4 mRNA and expression of IL-4 protein in primary human T cells.43 Polyphenol-containing compounds preferentially activated IL-4-secreting Th2 cells, thereby favouring IgE production.44 In this report, we add BPA and NP to the list of environmental components that increase allergic responses, as characterized by increased levels of IL-4 production in CD4+ T cells and higher levels of antigen-specific IgE in sera.
Our study demonstrated that BPA or NP, two widely used types of EDs, can stimulate a Ca2+/calcineurin/NF-AT signalling pathway, leading to the enhancement of IL-4 production in CD4+ T cells. These results suggest a possible enhancing mechanism of EDs such as BPA and NP on allergic responses. Because the ratio of Th1 and Th2 cells is closely correlated with the outcome of many diseases,45,46 controlling exposure to major sources of BPA and NP may protect patients from developing diseases caused by undesired Th2-dominated responses.
Acknowledgments
We thank Drs M. Howard and Y. K. Choe for their generous gift of valuable reagents, and Drs E. P. Cohen and H. J. Han for helpful discussions and critical reading of the manuscript. This study was supported by Korea Research Foundation Grant (KRF-1999-005-F00005).
Abbreviations
- BPA
bisphenol A
- ED
endocrine disruptor
- ELISA
enzyme-linked immunosorbent assay
- KLH
keyhole limpet haemocyanin
- NP
nonylphenol
- PMA
phorbol-12-myristate-13-acetate
- RT–PCR
reverse transcriptase–polymerase chain reaction
- Th2
T helper type 2
References
- 1.Moline JM, Golden AL, Bar-Chama N, et al. Exposure to hazardous substances and male reproductive health: a research framework. Environ Health Perspect. 2000;108:803–13. doi: 10.1289/ehp.00108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillette LJ, Jr, Crain DA, Rooney AA, Pickford DB. Organization versus activation: the role of endocrine disrupting contaminants (EDCs) during embryonic development in wildlife. Environ Health Perspect. 1995;103:157–64. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper RL, Kavlock RJ. Endocrine disruptors and reproductive development: a weight of evidence overview. J Endocrinol. 1997;152:159–66. doi: 10.1677/joe.0.1520159. [DOI] [PubMed] [Google Scholar]
- 4.Gray LE, Jr, Ostby J. Effects of pesticides and toxic substances on behavioral and morphological reproductive development: endocrine versus nonendocrine mechanisms. Toxicol Ind Health. 1998;14:159–84. doi: 10.1177/074823379801400111. [DOI] [PubMed] [Google Scholar]
- 5.Davis DL, Bradlow HL. Can environmental estrogens cause breast cancer? Sci Am. 1995;273:166–72. [PubMed] [Google Scholar]
- 6.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–86. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 7.Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonylphenol: an estrogenic xenobiotic released from modified polystylene. Environ Health Perspect. 1991;92:167–73. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrissey RE, George JD, Price CJ, Tyl RW, Marr MC, Kimmel CA. The developmental toxicity of bisphenol A in rats and mice. Fundam Appl Toxicol. 1987;8:571–82. doi: 10.1016/0272-0590(87)90142-4. [DOI] [PubMed] [Google Scholar]
- 9.Kanerva L, Estlander T, Keskinen H, Jolanki R. Occupational allergic airbone contact dermatitis and delayed bronchial asthma from epoxy resin revealed by bronchial provocation test. Eur J Dermatol. 2000;10:475–7. [PubMed] [Google Scholar]
- 10.Nethercott JR, Lawrence MJ. Allergic contact dermatitis due to nonylphenol ethoxylate (nonoxynol-6) Contact Dermatitis. 1984;10:235–9. doi: 10.1111/j.1600-0536.1984.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 11.Coyle AJ, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti IgE antibody. J Exp Med. 1996;183:1303–10. doi: 10.1084/jem.183.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eum SY, Haile S, Lefort J, Huerre M, Vargaftig BB. Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin-5-dependent bronchial hyperresponsiveness. Proc Natl Acad Sci U S A. 1995;92:12290–4. doi: 10.1073/pnas.92.26.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapsenberg ML, Hilkens CM, Jansen HM, Bos JD, Snijders A, Wierenga EA. Production and modulation of T-cell cytokines in atopic allergy. Int Arch Allergy Immunol. 1996;110:107–13. doi: 10.1159/000237274. [DOI] [PubMed] [Google Scholar]
- 14.Secrist H, Dekruyff RH, Umetsu DT. IL-4 production by CD4+ T cells from allergic individuals is modulated by antigen concentration and antigen-presenting cell type. J Exp Med. 1995;181:1081–9. doi: 10.1084/jem.181.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulay JL, Paul WE. The interleukin-4-related lymphokines and their binding to hematopoietin receptors. J Biol Chem. 1992;267:20525–8. [PubMed] [Google Scholar]
- 16.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–806. [PubMed] [Google Scholar]
- 17.Dekruyff RH, Fang Y, Wolf SF, Umetsu DT. IL-12 inhibits IL-4 synthesis in keyhole limpet hemocyanin-primed CD4+ T cells through an effect on antigen-presenting cells. J Immunol. 1995;154:2578–87. [PubMed] [Google Scholar]
- 18.Kim TS, Kim SH, Hwang SY. Injection with interleukin-4-secreting fibroblasts efficiently induces T helper type 2 cell-dominated immune response. Vaccine. 2000;18:2832–7. doi: 10.1016/s0264-410x(00)00075-x. [DOI] [PubMed] [Google Scholar]
- 19.Kim TS, Dekruyff RH, Rupper R, Maecker HT, Levy S, Umetsu DT. An ovalbumin-IL-12 fusion protein is more effective than ovalbumin plus recombinant IL-12 in inducing a T helper cell type 1-dominanted immune response and inhibiting antigen-specific IgE production. J Immunol. 1997;158:4137–44. [PubMed] [Google Scholar]
- 20.Kim TS, Chung SW, Kim SH, Kang BY. Therapeutic anti-tumor response induced with epitope-pulsed fibroblasts genetically engineered for B7.1 expression and IFN-γ secretion. Int J Cancer. 2000;87:427–33. doi: 10.1002/1097-0215(20000801)87:3<427::aid-ijc18>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase in a soluble extract from isolated mammalian nuclei. Nucl Acids Res. 1983;11:1475–89. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–70. [PubMed] [Google Scholar]
- 23.Hughes PJ, McLellan H, Lowes DA, et al. Estrogenic alkylphenols induce cell death by inhibiting testis endoplasmic reticulum Ca2+ pumps. Biochem Biophys Res Commun. 2000;277:568–74. doi: 10.1006/bbrc.2000.3710. [DOI] [PubMed] [Google Scholar]
- 24.Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402:18–23. doi: 10.1038/35037014. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler DJ, Parveen S, Pollock K, Williams RJ. Inhibition of sCD23 and immunoglobulin E release from human B cells by a metalloproteinase inhibitor, GI 129471. Immunology. 1998;95:105–10. doi: 10.1046/j.1365-2567.1998.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahy JV. Reducing IgE levels as a strategy for the treatment of asthma. Clin Exp Allergy. 2000;30:16–21. doi: 10.1046/j.1365-2222.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 27.Haas H, Falcone FH, Holland MJ, Schramm G, Haisch K, Gibbs BF, Bufe A, Schlaak M. Early interleukin-4. its role in the switch towards a Th2 response and IgE-mediated allergy. Int Arch Allergy Immunol. 1999;119:86–94. doi: 10.1159/000024182. [DOI] [PubMed] [Google Scholar]
- 28.Oettgen HC. Regulation of the IgE isotype switch: new insights on cytokine signals and the functions of epsilon germline transcripts. Curr Opin Immunol. 2000;12:618–23. doi: 10.1016/s0952-7915(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 29.Szabo SJ, Gold JS, Murphy TL, Murphy KM. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells. Roles for NF-Y and NF-Atc. Mol Cell Biol. 1993;13:4793–805. doi: 10.1128/mcb.13.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider G, Heinfling A, Klein-Hessling S, Schomberg C, Chuvpilo S, Serfling E. The inducible transcription factor NF-AT plays an important role in the activation of the murine interleukin-4 promoter. Immunobiology. 1995;193:268–72. doi: 10.1016/s0171-2985(11)80554-1. [DOI] [PubMed] [Google Scholar]
- 31.Rooney JW, Hoey T, Glimcher LH. Coordinate and cooperative roles for NF-AT and AP-1 in the regulation of the murine IL-4 gene. Immunity. 1995;2:473–83. doi: 10.1016/1074-7613(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 32.Luo C, Burgeon E, Carew JA, McCaffrey PG, Badalian TM, Lane WS, Hogan PG, Rao A. Recombinant NFAT1 (NFATp) is regulated by calcineurin in T cells and mediates transcription of several cytokine genes. Mol Cell Biol. 1996;16:3955–66. doi: 10.1128/mcb.16.7.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crabtree GR. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–4. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 34.Rao A, Luo C, Hogan PG. Transcription factors of the NF-AT family: regulation and function. Ann Rev Immunol. 1997;15:707–47. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 35.Weiss DL, Hural J, Tara D, Timmerman LA, Henkel G, Brown MA. Nuclear factor of activated T cells is associated with a mast cell interleukin-4 transcription complex. Mol Cell Biol. 1996;16:228–35. doi: 10.1128/mcb.16.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan SC, Brown MA, Willcox TM, Li SH, Stevens SR, Tara D, Hanifin JM. Abnormal IL-4 gene expression by atopic dermatitis T lymphocytes is reflected in altered nuclear protein interactions with IL-4 transcriptional regulatory element. J Invest Dermatol. 1996;106:1131–6. doi: 10.1111/1523-1747.ep12340181. [DOI] [PubMed] [Google Scholar]
- 37.Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–40. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 38.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK 506. Immunol Today. 1992;13:136–42. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 39.Long X, Burke KA, Bigsby RM, Nephew KP. Effects of the xenoestrogen bisphenol A on expression of vascular endothelial growth factor (VEGF) in the rat. Exp Biol Med. 2001;226:477–83. doi: 10.1177/153537020122600514. [DOI] [PubMed] [Google Scholar]
- 40.Yoo SD, Shin BS, Lee BM, Lee KC, Han SY, Kim HS, Kwack SJ, Park KL. Bioavailability and mammary excretion of bisphenol A in Sprague–Dawley rats. J Toxicol Environ Health A. 2001;64:417–26. doi: 10.1080/152873901753170740. [DOI] [PubMed] [Google Scholar]
- 41.Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM., Jr The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci. 2000;54:3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 42.Takafuji S, Nakagawa T. Air pollution and allergy. J Invest Allergol Clin Immunol. 2000;10:5–10. [PubMed] [Google Scholar]
- 43.Bommel H, Li-Weber M, Serfling E, Duschl A. The environmental pollutant pyrene induces the production of IL-4. J Allergy Clin Immunol. 2000;105:796–802. doi: 10.1067/mai.2000.105124. [DOI] [PubMed] [Google Scholar]
- 44.Baum CG, Szabo P, Siskind GW, Becker CG, Firpo A, Clarick CJ, Francus T. Cellular control of IgE induction by a polyphenol-rich compound: Preferential activation of Th2 cells. J Immunol. 1990;145:779–84. [PubMed] [Google Scholar]
- 45.Deisinger PJ, Hill TS, English JC. Human exposure to naturally occurring hydroquinone. J Toxicol Environ Health. 1996;47:31–46. doi: 10.1080/009841096161915. [DOI] [PubMed] [Google Scholar]
- 46.Seymour BW, Pinkerton KE, Friebertshauser KE, Coffman RL, Gershwin LJ. Second-hand smoke is an adjuvant for T helper-2 responses in a murine model of allergy. J Immunol. 1997;159:6169–75. [PubMed] [Google Scholar]