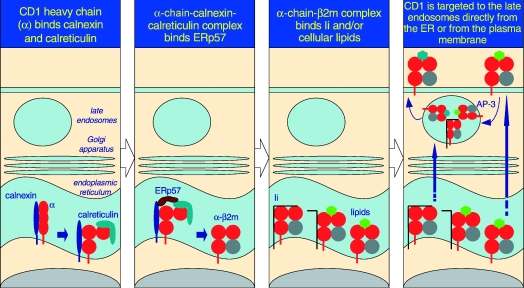
Figure 1.
The predicted pathway for CD1d assembly and intracellular trafficking. As it is a type I integral membrane glycoprotein, the folding and assembly of CD1d occur in the rough ER. Here, calnexin, calreticulin and ERp57 assist the folding of the CD1d α-chain. β2m associates with the folded α-chain, unlike MHC class I molecules, which bind calreticulin only after association with the light chain. The α-chain+β2m+ complex forms a structure receptive to Ii and/or resident lipids and glycolipids. Upon stable association, the CD1d (α-chain and β2m)–glycolipid complexes egress from the ER and negotiate the secretory pathway to the plasma membrane. Because of the Yxxφ internalization sequence within the cytosolic tail of CD1d, it is rapidly internalized into the late endosomes in an AP-3-dependent manner and recycled back to the plasma membrane. In contrast, Ii-associated CD1d may be directly targeted to the late endosomes, and thence egress to the cell surface. During its time in the late endosomes, lipids bound to CD1d in the ER are exchanged for another, presumably cellular, glycolipid that is presented to iNKT cells.