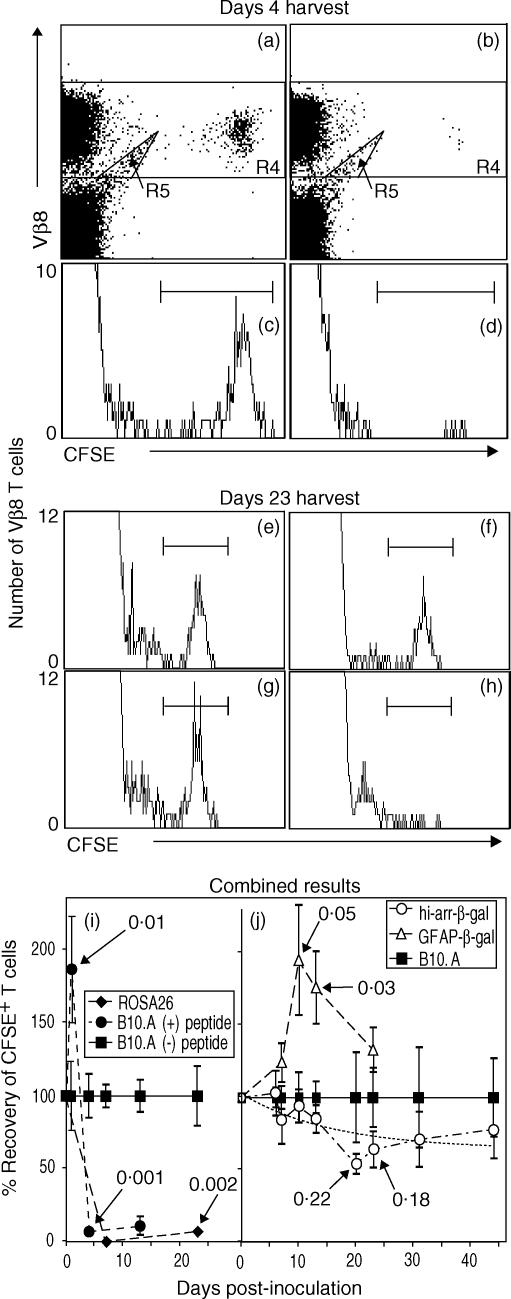
Figure 4.
Survival of β3 cells in control and transgenic (Tg) mice, demonstrating recognition of β-galactosidase (β-gal) in vivo by resting β3 cells. Splenocytes were harvested at the indicated time-points and analysed, by flow cytometry, for β3 cells [CD3+ CD8+ Vβ8+ 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE+) cells]. Representative analyses for harvests on day 4 (a–d) and day 23 (e–h) are shown. (a–d) Resting β3 cells were transferred to B10.A recipients on day 0. Peptide (300 µg) or saline was given on day 1, and spleens were harvested on day 4. Scatter plots were gated on lymphocyte-sized events that were further analysed for CD3+ CD8+ T cells. Positive events were then analysed for Vβ8+ CFSE-labelled events, as shown in (a) (no peptide) and (b) (with peptide). CFSE+ cells are clearly visible and were decreased in number following administration of peptide. Events in R4, excluding the artefact ‘tail’ in R5, were analysed on histograms (c, no peptide; d, with peptide) to provide the data for the combined experiments (i). No events were found in the CFSE+ region in mice not transferred with β3 cells, but the R5 ‘tail’ was still present, showing that it is not derived from the transferred cells (data not shown). (e–h) Recovery of β3 cells from control and Tg mice 23 days after transfer. The histograms were generated as described for panels (a)–(d), and show the relative changes in recovery. Recipients included: (e) B10.A; (f) hi-arr-β-gal; (g) GFAP-β-gal; and (h) ROSA26. Combined data are shown for mice expressing β-gal in non-immune-privileged sites (i) compared with expression in an immune-privileged site (hi-arr-β-gal mice) (j) For each time-point, the number of β3 cells recovered was normalized to the number recovered from normal B10.A mice at that same time-point (defined as 100%). A non-linear regression analysis for the hi-arr-β-gal mice is presented (dashed line, j). P-values for selected comparisons with β3-transferred B10.A controls are indicated. Each time-point in (i) and (j) represents a minimum of four mice, each analysed individually.