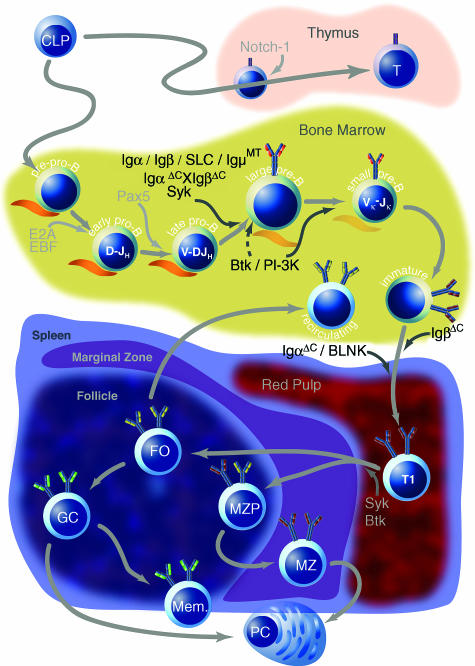
Figure 2.
Overview of B-cell development in postnatal mammalian life. Common lymphoid progenitors (CLPs) can migrate to the thymus where they become T cells in response to signalling through Notch-1, or they can remain in the bone marrow where they enter the B lineage as pre-pro-B cells. Possibly in response to Notch-1 down-regulation by Pax5, transcription factors EBF and E2A are up-regulated to initiate a B-cell-specific programme of gene transcription. DH–JH rearrangements occur at the early pro-B stage, and subsequent V–DJ rearrangements commence at the late pro-B stage. These stages require direct contact with bone marrow stromal cells, depicted in orange. Signalling molecules, whose absence results in a developmental block, are listed in black; dashed arrows indicate incomplete impairment. Appropriate signalling through the pre-B-cell receptor (BCR) (the surrogate light chain is depicted in orange) mediates heavy-chain allelic exclusion and induces several rounds of proliferation. Subsequently, at the small pre-B stage, light-chain rearrangements begin. Successfully rearranged heavy and light chains appear on the cell surface at the immature B-cell stage, after which B cells emigrate to the periphery. Newly immigrated transitional immature B cells (T1) probably undergo further differentiation into T2 and possibly T3 transitional immature B cells, although this is controversial. Eventually, transitional cells enter splenic follicles, where they differentiate further along the marginal zone (MZP, MZ) or follicular (FO) pathways, probably in response to the strength of signals received through the BCR. Follicular cells differentiate either into plasma cells (PCs) or germinal centre B cells (GC) in response to primary antigen challenge, whereupon they undergo somatic hypermutation, affinity maturation and class switch before they differentiate into memory cells. Marginal zone cells, which leave the splenic follicle to reside in the marginal zone, are also capable of differentiating into plasma cells in response to primary antigen exposure.