Abstract
Exogenous antigens are generally presented by Class II major histocompatibility (MHC) molecules. When administered with an adjuvant, however, they are capable of inducing a CD8+ T-cell response where antigen recognition is associated with Class I MHC. Accordingly, immunization with soluble ovalbumin (OVA) alone does not activate CD8+ cytotoxic T cells (CTL) but when given in complete Freund's adjuvant (CFA), or in formulations of a number of novel adjuvants, an OVA-specific CD8+ CTL response can be detected. We show in this report that immunization with soluble OVA mixed with heat-killed Mycobacterium vaccae, but not with other common pathogenic and saprophytic mycobacteria, can activate OVA-specific CD8+ CTL. An OVA-specific CTL response is detected when mice are immunized by either the intraperitoneal or intranasal route and their spleen cells are re-stimulated in vitro. Adjuvant activity of heat-killed M. vaccae is present in M. vaccae culture filtrate, in soluble protein components of whole M. vaccae and in the 65 kDa heat-shock protein (hsp) of M. vaccae. Mycobacterium vaccae has previously been shown to have no adverse side-effects in humans. The current results suggest that M. vaccae may be useful as an adjuvant for vaccines and other immunotherapies where CD8+ CTL responses to exogenous proteins are crucial.
Introduction
New generation vaccines are being developed which are better defined and offer advantages, such as lower toxicity, but they are often poorly immunogenic. Consequently, there is a need for new potent safe adjuvants or immunomodulators that are compatible with subunit or DNA vaccines. There are several classes of adjuvants, including aluminium salts, surface active reagents, polyanions, bacterial products and slow-release compounds. Aluminium adjuvants, together with calcium phosphate and a squalene formulation are the only adjuvants approved for human vaccine use. These approved adjuvants are not effective in stimulating cell-mediated immunity but rather stimulate a good antibody response.1 Successful vaccination against a number of infectious diseases requires both humoral and cell-mediated immunity and for some diseases, such as tuberculosis, protective immunity appears to be strongly associated with a T helper type 1 (Th1) response.2 In recent years there has been a search for adjuvants that may selectively promote a Th1 response and there is evidence that infectious organisms such as Leishmania3 and some mycobacteria4 can contain such adjuvant components.
Mycobacterium vaccae is a non-pathogenic mycobacterium found in the soil. It has been considered as a possible vaccine or immunotherapeutic agent for human tuberculosis5 although its efficacy, at present, is controversial.6,7 It is proposed that M. vaccae promotes a Th1 response, overcoming the pathogenic Th2 response of individuals with tuberculosis while providing cross-reactive epitopes to stimulate a protective immune response.7 In the mouse, immunization with a low dose of heat-killed M. vaccae reduces the mycobacterial load after challenge with M. tuberculosis.8 Both CD4+ and CD8+ T cells appear to be important in the protective immune response against M. tuberculosis.2,9 Immunization with heat-killed M. vaccae leads to, amongst other things, the stimulation of CD8+ T cells. These produce interferon-γ (IFN-γ) in response to macrophages infected with M. tuberculosis, stimulate the production of interleukin-12 (IL-12) and specifically lyse M. tuberculosis-infected macrophages.10 Thus M. vaccae is capable of stimulating a cytotoxic response associated with type 1 cytokines (TC1) immune response to antigens shared with M. tuberculosis.
Exogenous antigens, such as those present in the heat-killed M. vaccae preparation, are usually not presented via the major histocompatibility complex (MHC) Class I pathway; however, there are exceptions.11 Macrophages can regurgitate processed antigen as peptides12 or transfer antigens from phagosomes into the cytosol so that endogenous and exogenous antigens use a final common pathway for Class I presentation.13,14 Exogenous antigens can prime CD8+ cytotoxic T cells (CTL) when they are administered with an adjuvant15–20 although stimulation of CTL by native protein appears to depend on the characteristics of the protein as well as the adjuvant.21–23 Heat-killed M. vaccae may contain adjuvants together with antigens common to M. tuberculosis which together stimulate the production of M. tuberculosis-specific CD8+ CTL.
In this report we have used the CTL response to ovalbumin (OVA) to determine whether M. vaccae can function as an adjuvant to stimulate CD8+ CTL responses to other protein antigens. We observe that heat-killed M. vaccae, but not other heat-killed mycobacteria such as M. bovis bacillus Calmette–Guèrin (BCG) or M. tuberculosis and other pathogenic and saprophytic mycobacteria, can induce a CD8+ CTL response when given to mice in conjunction with OVA. Furthermore, heat-killed M. vaccae can function as an adjuvant for stimulation of an immune response through the mucosa of the airways. The significance of these findings to current concepts in immunology and to the development of a more effective adjuvant or immunotherapeutic agent suitable for use in humans is discussed.
Materials and methods
Antigens and adjuvants
Purified chicken ovalbumin (OVA Grade VI), muramyl dipeptide (N-acetylmuramyl-l-alanyl-d-isoglutamine) and trehalose 6, 6′-dimycolate from M. tuberculosis were purchased from Sigma Chemical Co. (St. Louis, MO). Complete Freund's adjuvant (CFA) was purchased from Difco (Detroit, MI). OVA was checked by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) for low molecular weight impurities, which were found only to be present in trace amounts.
Mycobacterium vaccae (ATCC 15483), M. smegmatis (ATCC 27199), M. phlei (ATCC 11758), M. tuberculosis H37Rv (ATCC 27294) and M. bovis BCG were obtained from AgResearch Wallaceville, New Zealand and grown by standard procedures. Culture filtrates of M. vaccae were supernatants from cultures of M. vaccae in 7H9 medium passed through a 0·45-μm filter. Heat-killed M. vaccae was prepared from the mycobacterial pellet resuspended in phosphate-buffered saline (PBS) at 10 mg/ml (equivalent to 1010 organisms per ml) and autoclaved for 15 min at 120°. To produce M. vaccae components, M. vaccae culture filtrate was concentrated by ultrafiltration [10 kDa molecular weight cut off (MWCO)], precipitated in 10% trichloroacetic acid (TCA), redissolved in 0·1 m Tris buffer (pH 8), re-precipitated with acetone, dissolved in water and finally re-precipitated in chloroform/methanol (2 : 1) at a ratio of 6 volumes chloroform/methanol to one volume of water. A delipidated and deglycolipidated form of M. vaccae (DD-M. vaccae) was prepared by sonicating autoclaved M. vaccae, and resuspending the pellet after centrifugation in 100 ml chloroform/methanol (2 : 1). After incubation at room temperature for 1 hr the material was centrifuged, re-extracted in chloroform/methanol, re-centrifuged and the dried pellet was resuspended in PBS. Glycolipids were removed by refluxing in 50% v/v ethanol for 2 hr and collecting the insoluble material by centrifugation. This extraction was repeated twice. Protein extracts were made by treating DD-M. vaccae with 2% SDS in PBS for 2 hr at 56°. The protein extraction was also repeated twice and SDS was removed from the extract by precipitation at 4°. After removal of the SDS precipitate the extracted proteins were precipitated with acetone, incubated at −20° for 2 hr, collected by centrifugation, dried and re-dissolved in PBS.
Recombinant M. vaccae heat-shock protein 65 (hsp 65) was obtained as follows. Serum, from M. vaccae-immunized cynomolgus monkeys, was used to probe a BamHI lambda-ZAP (Stratagene, La Jolla, CA) genomic library of M. vaccae. An hsp 65 clone was verified as plaque pure at the tertiary screen, excised to a phagemid clone and sequenced to reveal a 2·2-kilobase (kb) partial clone of a hsp 65 homologue containing the 3′ end of the M. vaccae hsp 65 gene. To obtain the full-length clone, two primers were designed, one complementary to the 5′ end of the partial sequence, the other complementary to the 5′ end sequence of hsp 65 from M. tuberculosis, M. paratuberculosis and M. leprae. These primers were used to amplify a 642-nucleotide fragment which was cloned into Bluescript (Stratagene, La Jolla, CA), linearized and ligated to the partial M. vaccae hsp 65 2·2-kb BamHI fragment to assemble the full-length hsp 65 gene in PBS. The full-length gene was cloned directionally and in frame into pET16 with a modified polylinker and expressed as a 6xHis–hsp 65 fusion protein. The protein was produced from recombinant Escherichia coli both as a soluble protein and as insoluble inclusion bodies. These were combined, after denaturing in 6 m guanidine (Gdn)HCl, and purified by chromatography on nickel-chelating Sepharose FF. The column was washed with binding buffer (6 m urea/0·5 m NaCl/20 mm Tris–HCl pH 8·0), with binding buffer containing 0·5% sodium deoxycholate, and with binding buffer containing 20 mm imidazole. The hsp 65 was eluted with binding buffer containing 300 mm imidazole, and was refolded by dialysis against decreasing concentrations of urea in 20 mm Tris–HCl pH 7·5. More than 90% of the protein was shown, by analytical SDS–PAGE, to be present as a single band of apparent MW 61 kDa.
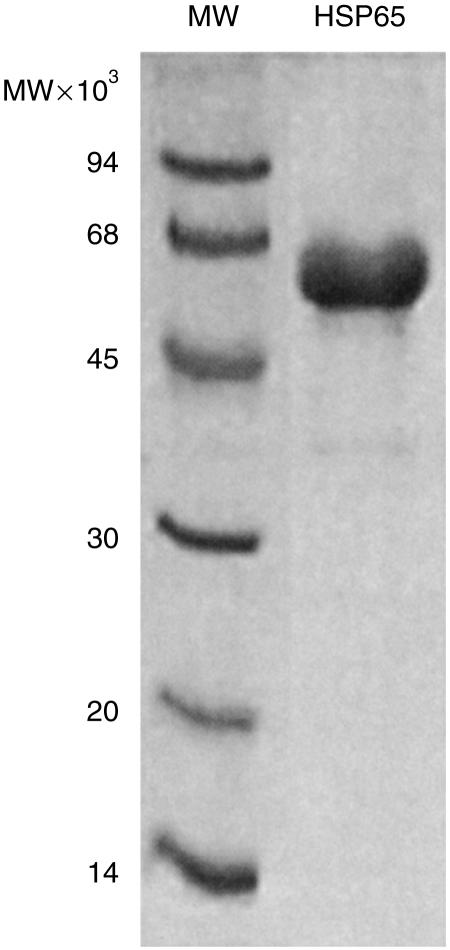
The expression of genes in recombinant bacteria may lead to contamination of the purified proteins with bacterial endotoxin (lipopolysaccharide; LPS). In this case, the histidine-tagged recombinant-derived protein was purified by affinity chromatography on nickel-chelating Sepharose. While bound to the column, the protein was washed with buffer containing sodium deoxycholate to remove bacterial contaminants, including LPS. Residual endotoxin levels were assayed by the colorimetric Limulus amoebocyte lysate assay and were found to be less than 100 U/mg protein. A gel of the purified M. vaccae hsp is shown in Fig. 1. Other recombinant proteins of M. vaccae including the homologues of the antigen 85 (Ag 85) A, B and C complex of M. tuberculosis were derived as detailed elsewhere24 and purified in the same way. Endotoxin levels in these preparations were also less than 100 U/mg protein.
Figure 1.
Analytical SDS–PAGE of purified M. vaccae HSP65. A sample of purified M. vaccae HSP65 (approximately 5 µg) was analysed by SDS–PAGE (12·5% acrylamide/0·33% Bis) and the gel was stained with Coomassie blue R250.
Protease treatment
Soluble protein extracts of DD-M. vaccae were treated with Pronase E (Merck, Darmstadt, Germany). The equivalent of 20 µg protein was incubated with 20 µg Pronase E at 37° for 4 hr. The enzyme was heat-inactivated at 95° for 10 min before samples were mixed with OVA for injection into mice.
Animals and immunization
Specific pathogen-free female 7–10-week-old C57BL/6J (H-2b) mice were obtained from the Department of Laboratory and Animal Science, University of Otago, Dunedin, New Zealand, or from the animal facility at Genesis R & D Corp. Ltd, Auckland, New Zealand. Groups of mice (three to five mice per group) were immunized intraperitoneally with 100 µg OVA in PBS, adjuvant, or mixed with mycobacterial preparations. For heat-killed mycobacterial preparations, 1 mg wet weight was mixed with 100 µg OVA as this dose of heat-killed M. vaccae had been shown previously to stimulate a CTL response to mycobacterial antigens10 and natural killer cell activity.25 For intranasal immunizations mice were anaesthetized with 4–6 mg ketamine/0·025% xylazine per 20 g mouse. OVA or OVA mixed with 500 µg autoclaved M. vaccae in a volume of 40 µl was delivered one drop at a time to each nostril. This dose of M. vaccae was more convenient to administer by the intranasal route.
In vitro stimulation of CTL
Spleens were removed 9–10 days after immunization, the cells of each group were pooled and 3·5 × 107 spleen cells were cultured with 3 × 106 E.G7-OVA11 which had previously been γ-irradiated (20 000 rads) or treated with mitomycin C (Sigma Chemical Co.) at 20 µg/ml for 2 hr. Cultures were set up in a total volume of 10 ml Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal calf serum, 5 × 10−5 m 2-mercaptoethanol, 110 mg/l sodium pyruvate, 36 mg/l l-arginine, 116 mg/l folic acid, 60 mg/l penicillin, 100 mg/l streptomycin and 2 mm glutamine (tissue culture medium). After 6 days at 37° in 5% C02 in air, the cytotoxic activity of effector cells was assayed.
Cytotoxicity assay
EL4 and E.G7-OVA target cells were labelled with 100–200 µCi 51Cr-labelled sodium chromate at 37° for 45 min. After washing, 5 × 103 51Cr-labelled target cells were incubated with effector cells at graded dilutions in 96-well V-bottomed plates. Supernatants were harvested after 5 hr and radioactivity was determined in a Micro Beta Plus liquid scintillation counter (Wallac, Finland). The percentage specific lysis was calculated according to the formula: [(c.p.m. released by CTL − c.p.m. released spontaneously) / (total c.p.m. taken up − c.p.m. released spontaneously)] × 100, where c.p.m. are counts per minute.
Spontaneous release was less than 14% of total c.p.m. taken up. Standard deviations of triplicate estimations did not exceed 4% specific lysis. Lytic units (LU) were calculated from the concentration of effector cells which gave 50% specific lysis and were expressed as log10. For example if the fraction of a culture containing effector cells that resulted in 50% specific lysis was 0·033, there were 30·3 lytic units generated per culture (1/0·033) which is 1·48 log10 LU. Lytic units were only calculated from experiments where a least three data points were on the straight line part of the plot of percentage specific lyis against log10 concentration of effector cells. Geometric means of lytic units from replicate experiments were expressed with standard deviations.
CD8+ T-cell depletion
Spleen cell cultures were depleted of CD8+ T cells by antibody-mediated complement lysis or by passage through a T-cell separation column. Cells were treated with 1 µg/ml anti-CD8 (clone 536.72, Pharmingen, San Diego, CA) for 30 min on ice, washed once and incubated with low toxicity rabbit complement (Serotec, Oxford, UK) at a final dilution of one in five. After incubation at 37° for 45 min, dead cells were removed by centrifugation over Ficoll and washed three times. Alternatively, cells were loaded onto a T-cell separation column which depleted CD8+ T cells (R & D Systems, Minneapolis, MN) and run according to the manufacturer's instructions. After depletion, cells were tested for residual CD8+ cells by flow cytometry and assayed for cytotoxic activity.
Results
Induction of OVA-specific CTL by OVA delivered with M. vaccae
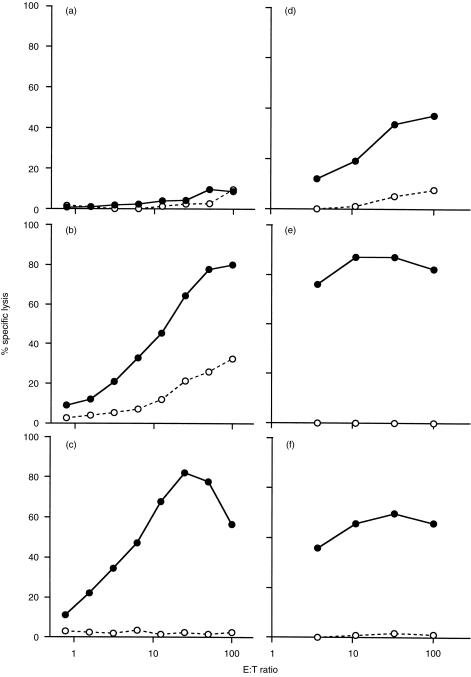
Spleen cells from C57BL/6J mice immunized with OVA in various formulations were examined for the development of OVA-specific CTL responses. The spleen cells were cultured with irradiated E.G7-OVA stimulator cells for 6 days and then assayed for cytotoxic activity on E.G7-OVA and EL4 target cells. The results shown in Fig. 2 are from two separate experiments. In the first experiment mice immunized with OVA mixed with 1 mg heat-killed M. vaccae generated a highly specific OVA CTL response (Fig. 2c), whereas a CTL response was not detectable when mice were immunized with native OVA (Fig. 2a). Treatment with heat-killed M. vaccae alone did not stimulate a CTL response (data not shown). As expected, immunization with OVA in CFA generated OVA-specific CTL but there was some nonspecific lysis of EL4 target cells (Fig. 2b). No response was detected after immunization with OVA in IFA (data not shown). In a second experiment two other known adjuvants derived from mycobacteria, muramyl dipeptide and trehalose 6, 6′-dimycolate also stimulated an OVA-specific CTL response (Fig. 2d,e). In the second experiment, in which heat-killed M. vaccae (Fig. 2f) was used as the positive control, the maximum percentage specific lysis plateaued at 60% whereas in the results of the experiment shown in Fig. 2(c) it reached nearly 80%. To demonstrate that heat-killed M. vaccae does give reproducible adjuvant activity, even though the percentage specific lysis sometimes reaches a plateau at different levels, the results of 10 additional separate experiments were analysed and results expressed as mean lytic units are shown in Table 1. These results confirm that although exogenous OVA primes CTL when injected in a water-in-oil emulsion containing M. smegmatis (CFA), OVA mixed with M. vaccae or known mycobacterial adjuvant components in the absence of oil is sufficient for the development of OVA-specific CTL.
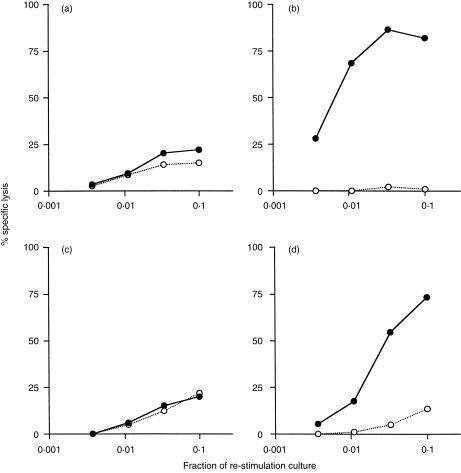
Figure 2.
Induction of OVA-specific CTL response by OVA delivered with M. vaccae. C57BL/6J mice were immunized i.p. with (a) OVA alone, (b) OVA in CFA, (c) OVA plus 1 mg heat-killed M. vaccae; or with (d) OVA plus 50 µg muramyl dipeptide, (e) OVA plus 50 µg trehalose 6, 6′-dimycolate, or (f) OVA plus 1 mg heat-killed M. vaccae. Spleens were removed 10 days later and splenocytes were stimulated in vitro with E.G7-OVA cells for 6 days. The cytotoxic activities were measured using 51Cr-labelled E.G7-OVA (•), or EL4 (○). Per cent specific lysis at various effector to target ratios are shown and represent two separate experiments.
Table 1.
Adjuvant activity of different components of M. vaccae
| Adjuvant | Lytic units (mean ± SD)§ |
|---|---|
| 1 mg heat killed M. vaccae | 2·36 ± 0·26 |
| (n = 10)* | |
| 100 µg M. vaccae culture supernatant | 2·27 ± 0·30 |
| (n = 4)† | |
| M. vaccae hsp65 | 1·48 ± 0·03 |
| (n = 3)‡ |
Number of experiments using four different batches of heat-killed M. vaccae;
number of experiments using four different batches of M. vaccae culture supernatant;
number of experiments using two different batches of M. vaccae hsp65;
geometric mean of log10lytic units per culture using EG.7-OVA target cells with standard deviation.
CTL induced by OVA and M. vaccae are CD8+
To establish whether the CTL response stimulated by M. vaccae was mediated by CD8+ CTL, spleen cell cultures from immunized mice were treated with anti-CD8 antibody and complement prior to assay for CTL activity. After CD8+ T-cell depletion, which did not totally deplete all CD8+ T cells, OVA-specific CTL activity was reduced from 2·72 to 1·48 log10 LU per 3·5 × 107 cultured spleen cells. Spleen cells, when passed through a column that removed > 95% CD8+ T cells, lost all detectable OVA-specific CTL activity (Table 2).
Table 2.
Effect of depletion of CD8+ cells on CTL activity after immunization with OVA mixed with M. vaccae
| Lytic units per culture | |||
|---|---|---|---|
| Treatment* | % CD8+ cells† | EG.7-OVA targets | EL4 targets |
| C′ | 17·2 | 2·72 ± 0·02‡ | < 0·1 |
| Anti-CD8 + C′ | 9·6 | 1·48 ± 0·03 | < 0·1 |
| Column depletion | < 5 | < 0·1 | < 0·1 |
Spleen cells from immunized mice were challenged in vitro with irradiated EG.7-OVA cells and 6 days later were depleted of CD8+ T cells before assay for CTL activity;
percentage of CD8+ T cells determined by flow cytometry;
geometric means and standard deviation of log10 lytic units from triplicate assays.
Other killed mycobacteria do not induce OVA-specific CTL
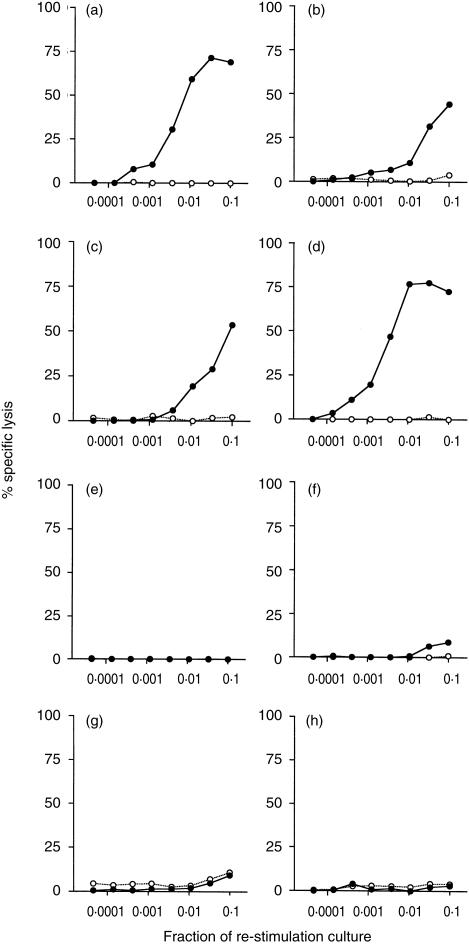
To investigate whether other common heat-killed mycobacteria alone can contribute to the development of an OVA-specific CTL response, C57BL/6J mice were immunized with OVA mixed with 1 mg heat-killed M. tuberculosis, M. bovis BCG, M. phlei, or M. smegmatis. Only mice immunized with OVA in combination with various doses of M. vaccae or M. vaccae culture filtrate developed OVA-specific CTL (Fig. 3). A dose of 1 mg heat-killed M. vaccae gave 2·10 log10 LU per culture whereas although 10 µg heat-killed M. vaccae was sufficient to stimulate a specific CTL response (Fig. 3c) only 1·09 log10LU per culture were generated. Heat-killed M. leprae was also unable to induce an OVA CTL response (data not shown). In a repeat experiment spleen cells from mice immunized with OVA administered with 1 mg heat-killed M. vaccae generated 2·05 log10LU/culture whereas spleen cells from mice immunized with OVA administered with other heat-killed mycobacteria did not generate a CTL response.
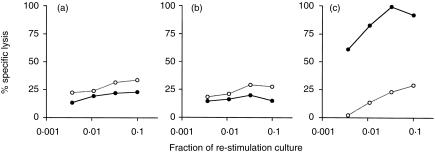
Figure 3.
Heat-killed M. tuberculosis does not induce OVA-specific CTL. C57BL/6J mice were immunized i.p. with OVA mixed with (a) 1 mg heat-killed M. vaccae, (b) 100 µg heat-killed M. vaccae, (c) 10 µg heat-killed M. vaccae, (d) 100 µg M. vaccae culture supernatant, (e) 1 mg heat-killed M. tuberculosis, (f) 1 mg heat-killed M. bovis BCG, (g) 1 mg heat-killed M. phlei, or (h) 1 mg heat-killed M. smegmatis. Spleens were removed 10 days later and splenocytes were stimulated in vitro with E.G7-OVA cells for 6 days. The cytotoxic activities were measured using 51Cr-labelled E.G7-OVA (•), or EL4 (○). The results shown are the per cent specific lysis at various dilutions of effector cells. One representative experiment of two is shown.
M. vaccae components
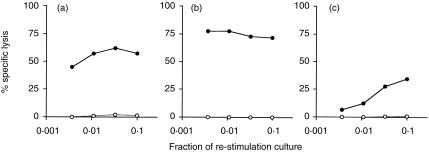
To determine which components of M. vaccae were involved in the adjuvant effect and to eliminate known lipid mycobacterial adjuvants, lipids and proteins were extracted from autoclaved M. vaccae. DD-M. vaccae elicited a CTL response comparable to that caused by the amount of heat-killed whole M. vaccae from which it was derived (data not shown). Protein from DD-M. vaccae was also highly active in eliciting a CTL response to soluble OVA and this was considerably reduced by treatment with Pronase E (Fig. 4b,c). A major component of the soluble M. vaccae protein ran at 65 kDa on a gel and protein sequence data showed homology to the 65 kDa hsp of M. tuberculosis. Consequently, the 65 kDa hsp from M. vaccae was cloned, sequenced, expressed in E. coli and tested for its ability to provide adjuvant activity for the stimulation of OVA-specific CTL in vivo. This protein displayed adjuvant activity that resulted in the generation of a highly specific CTL response to OVA (Fig. 5d) (1·48 log10LU per culture against EG.7-OVA target cells). Another recombinant protein from M. vaccae, the homologue of the M. tuberculosis Ag 85A protein, did not induce a cytotoxic response to OVA (Fig. 5a). Two further experiments were carried out using hsp 65 and mean LU/culture from the three experiments are shown in Table 1. In these experiments, seven other recombinant proteins from M. vaccae were shown not to exhibit adjuvant activity for CTL responses to OVA. They included the homologues of Ag 85B and Ag 85C of M. tuberculosis and GV7, GV14, GV22, GV28 and GV29.24
Figure 4.
Adjuvant activity is present in soluble proteins extracted from M. vaccae C57BL/6J mice were immunized i.p. with OVA mixed with (a) 1 mg heat-killed M. vaccae, (b)10 µg soluble protein extracted from M. vaccae, or (c) 10 µg soluble protein extracted from M. vaccae and treated with Pronase E. Spleens were removed 10 days later and splenocytes were stimulated in vitro with E.G7-OVA cells for 6 days. The cytotoxic activities were measured using 51Cr-labelled E.G7-OVA (•), or EL4 (○). The results shown are the per cent specific lysis at various dilutions of effector cells. One representative experiment of two is shown.
Figure 5.
Mycobacterium vaccae hsp65 has adjuvant activity. C57BL/6J mice were immunized i.p. with (a) OVA mixed with 50 µg M. vaccae Ag 85A, (b) OVA mixed with 1 mg heat-killed M. vaccae, (c) 50 µg M. vaccae hsp65, or (d) OVA mixed with 50 µg M. vaccae hsp65. Spleens were removed 10 days later and splenocytes were stimulated in vitro with E.G7-OVA cells for 6 days. The cytotoxic activities were measured using 51Cr-labelled E.G7-OVA (•), or EL4 (○). The results shown are the percent specific lysis at various dilutions of effector cells. One representative of three is shown.
Immunization at mucosal surfaces
The ability of M. vaccae to elicit a CTL response to OVA after immunization by the intranasal route was investigated. Mice were immunized intranasally with 100 µg OVA mixed with 500 µg heat-killed M. vaccae and their spleen cells were re-stimulated in vitro with mitomycin C-treated E.G7-OVA cells. Neither OVA nor M. vaccae alone could induce an OVA-specific CTL response, whereas when they were combined, a strong CTL response was detected (Fig. 6). The specificity of the response as indicated by the lysis of EL4 target cells was slightly less than that observed when immunization was via the intraperitoneal route. The results demonstrate that M. vaccae can provide adjuvant activity for immunization at mucosal surfaces.
Figure 6.
Heat-killed M. vaccae as a mucosal adjuvant. C57BL/6J mice were immunized intranasally with (a) OVA (b) 500 µg heat-killed M. vaccae, or (c) OVA mixed with 500 µg heat-killed M. vaccae. Spleens were removed 10 days later and splenocytes were stimulated in vitro with E.G7-OVA cells for 6 days. The cytotoxic activities were measured using 51Cr-labelled E.G7-OVA (•), or EL4 (○). The results shown are the per cent specific lysis at various dilutions of effector cells. One representative experiment of three is shown.
Discussion
An adjuvant is required for inducing a CTL response to peptides or proteins. Consequently, they have been incorporated into adjuvant vehicles such as liposomes,16,19 DNA26, formulated with or without oil-based adjuvants,17,18,20 or fused with hsp 70.27 The results of this study show that by simply mixing OVA with heat-killed M. vaccae, it is possible to stimulate an OVA-specific CD8+ CTL response.
It is well known that mycobacteria contain compounds with immunonstimulant activity. The first microbial products to be systematically investigated as adjuvants were components of M. tuberculosis. The trehalose 6,6′-dimycolate glycolipid or cord factor and forms of muramyl dipeptide are two that have been extensively studied.28 Two such compounds were shown to act as adjuvants in the stimulation of an OVA-specific CTL response by soluble OVA (Fig. 2). Live intact mycobacteria can act as highly effective adjuvants and recombinant BCG, expressing human immunodeficiency virus (HIV) genes, is currently being tested as a potential vaccine for HIV.29 Live BCG can stimulate a CTL response30 but when killed appears to be inactive both in stimulating CD8+ CTL specific for M. tuberculosis-infected macrophages (M. Skinner unpublished) and as an adjuvant for the induction of OVA-specific CTL (Fig. 3). Similarly, live M. tuberculosis can stimulate CD8+ CTL2 but killed M. tuberculosis can neither induce M. tuberculosis-specific CD8+ CTL (M. Skinner unpublished) nor act as an adjuvant for the generation of OVA-specific CTL18 (Fig. 3). Although purified components of M. tuberculosis do have adjuvant activity (Fig. 2) they may not be present in high enough concentrations or in an appropriate form in the heat-killed preparations of whole mycobacteria used in this study. A number of other common slow- and fast-growing heat-killed mycobacteria, were also inactive as adjuvants for induction of OVA-specific CTL responses (Fig. 3). In contrast, heat-killed M. vaccae stimulates CTL which will specifically lyse M. tuberculosis-infected target cells10 and can act as an adjuvant for a CTL response to an unrelated protein, OVA. (Table 1, Figs 2, 3).
In order to determine which components of M. vaccae contain adjuvant activity, M. vaccae was treated to remove lipids, glycolipids and soluble proteins. Although there was activity in insoluble components, it was clear that activity was also present in one or more proteins (Fig. 4). Adjuvant activity was almost entirely depleted from the soluble proteins of M. vaccae after pronase treatment. Protein adjuvants are uncommon, with the exception of hsp, which can bind small peptides and stimulate tumour-specific31 and viral-specific CTL.32 CTL responses to a whole protein, for example OVA, have been generated only when a fusion protein between OVA and a hsp is used for immunization.27 In this work M. vaccae was simply mixed with OVA immediately prior to immunization. Under these conditions of association it was not possible to generate a CTL response with the CTL immunodominant peptide SIINFEKL (OVA257−264) recognized in association with H2Kb (data not included), as CTL responses generated with hsp–peptide combinations require special conditions of association.32 Two recombinant polypeptides from M. vaccae were tested for adjuvant activity and a CTL response was detected using hsp 65 but not using Ag 85A. Both polypeptides had less than 100 U/mg protein LPS and seven other recombinant polypeptides from M. vaccae prepared in the same way did not exhibit this type of adjuvant activity (data not included). Consistent results were obtained using the purified recombinant hsp 65 as an adjuvant with less variability between replicate experiments than obtained using whole heat-killed M. vaccae or M. vaccae culture supernatant (Table 1).
IL-12 can enhance priming of CTL to exogenous antigens.33 Both M. vaccae hsp 65 and Ag 85A were good stimulators of IL-12 production from macrophages (data not shown), but they may have other differential effects on macrophages or other antigen-presenting cells. It has recently been reported that hsps can activate monocyte-derived macrophages through a CD14-dependent pathway, which results in an increase in the inflammatory cytokines IL-1β, IL-6 and tumour necrosis factor-α (TNF-α).34,35 Preliminary studies on human peripheral blood mononuclear cells indicate that M. vaccae hsp 65, like other Th1-inducing adjuvants including CpG oligodinucleotides and Monophosphoryl lipid A (MPL), stimulates production of IL-1β and TNF-α as well as that of IL-12.24 The pathway by which hsps stimulate cytokine production presumably involves Toll-like receptors (TLR) but this has yet to be shown. Activation via a CD14 independent pathway can leads to an increase in TNF-α alone.35 Although not yet demonstrated it is likely that a set of inflammatory chemokines are also stimulated which could modulate the migration of antigen-presenting cells and T cells. Thus hsps may have a dual role as both chaperone and cytokine and could have an important function in initiating immune responses.
It is unlikely that hsp 65 is the sole component of the protein adjuvant activity of M. vaccae as preliminary data suggests that there are additional polypeptides derived from M. vaccae that can function as adjuvants for CTL responses.24 There are to date six known members of the TLR family of receptors which may recognize a family of cytokine-like ligands that participate in host immune responses.36 Gram-negative LPS uses TRL-2 while the mycobacterial product lipoarabinomannan utilizes TLR-4 to initiate intracellular signals.37 Other mycobacterial components, may also mediate cellular activation via TLRs. It is likely that proteins as well as lipid-containing molecules may be ligands for TLR as the Toll ligand in the fly is a protein. Further studies are underway to investigate the effect of M. vaccae and recombinant polypeptides from M. vaccae on antigen-presenting cells.
Mycobacterium vaccae also has the ability to elicit an immune response when immunization is via the intranasal route (Fig. 5) demonstrating that it can act at a mucosal surface. There are few mucosal adjuvants and none at present registered for human use. The cholera toxin B subunit can induce mucosal CTL immune responses38 but is toxic. Mycobacterium vaccae is a potent immunomodulator, inducing strong Th1-type immune responses39 even during an established allergic Th2 response, leading to the inhibition of IL-5 and immunoglobulin E (IgE) production.40 It is a good inducer of IL-12 production,10,24 a property which may contribute to its ability to induce both Th1 and TC1 immune responses. Thus, in addition to providing a new mucosal adjuvant, the observation that heat-killed M. vaccae can act at respiratory surfaces makes it a good candidate for the development of new treatments for asthma and allergic rhinitis.
There are a number of novel adjuvants that have the ability to induce Class I-restricted CD8+ CTL responses after immunization with new generation vaccines such as proteins. However, some of these may be toxic or cause adverse reactions in humans. Heat-killed M. vaccae is safe, easy to produce and well-tolerated in humans.41,42 Although killed M. vaccae was originally proposed for tuberculosis immunotherapy it may prove to be a more useful treatment for other diseases such as prostate cancer42 and psoriasis43 and for new generation vaccines intended to stimulate cytotoxic responses mediated by CD8+ T cells.
Acknowledgments
We are grateful for Drs J. D. Watson and B. M. Buddle for critically reading the manuscript.
Abbreviations
- BCG
Mycobacterium bovis bacillus Calmette–Guèrin
- CFA
complete Freund's adjuvant
- CTL
cytotoxic T lymphocytes
- DD
delipidated and deglycolipidated
- DMEM
Dulbecco's modified Eagle's medium
- hsp
heat-shock protein
- IL-5
interleukin-5
- IL-12
interleukin-12
- IFN
interferon
- kDa
kilodalton
- MHC
major histocompatibility
- OVA
ovalbumin
- PBS
phosphate-buffered saline
- TCA
trichloroacetic acid
References
- 1.Singh M, O'Hagan D. Advances in vaccine adjuvants. Nature Biotech. 1999;17:1075–81. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 2.Orme IM, Miller ES, Roberts AD. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1993;167:1481–97. [PubMed] [Google Scholar]
- 3.Skeiky YA, Kennedy M, Kaufman D, et al. LeIF. a recombinant Leishmania protein that induces an IL-12-mediated Th1 cytokine profile. J Immunol. 1998;161:6171–9. [PubMed] [Google Scholar]
- 4.Yip HC, Karulin AY, Tary-Lehmann M, et al. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–9. [PubMed] [Google Scholar]
- 5.Stanford JL, Stanford CA, Rook GAW, Grange JM. Immunotherapy for tuberculosis. Investigative and practical aspects. Clin Immunother. 1994;1:430–40. [Google Scholar]
- 6.Durban Immunotherapy Trial Group. Immunotherapy with Mycobacterium vaccae in patients with newly diagnosed pulmonary tuberculosis: a randomised controlled trial. Lancet. 1999;354:116–19. 10.1016/s0140-6736(98)10448-8. [PubMed] [Google Scholar]
- 7.Dlugovitzky D, Bottasasso O, Dominino JC, Valentini E, Hartopp R, Singh M, Stanford C, Stanford J. Clinical and serological studies of tuberculosis patients in Argentina receiving immunotherapy with Mycobacterium vaccae (SRL 172) Respir Med. 1999;93:557–62. doi: 10.1016/s0954-6111(99)90155-5. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997;65:3317–27. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–17. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skinner MA, Yuan S, Prestidge R, Chuk D, Watson JD, Tan PLJ. Immunization with heat-killed Mycobacterium vaccae stimulates CD8+ cytotoxic T cells specific for macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1997;65:4525–230. doi: 10.1128/iai.65.11.4525-4530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–85. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 12.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–33. [PubMed] [Google Scholar]
- 13.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90:4942–6. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–6. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 15.Newman MJ, Wu JY, Gardner BH, Munroe KJ, Leombruno D, Recchia J, Kensil CR, Coughlin RT. Saponin adjuvant induction of ovalbumin-specific CD8+ cytotoxic T lymphocyte responses. J Immunol. 1992;148:2357–62. [PubMed] [Google Scholar]
- 16.Zhou F, Huang L. Monophosphoryl lipid A enhances specific CTL induction by a soluble protein antigen entrapped in liposomes. Vaccine. 1993;11:1139–44. doi: 10.1016/0264-410x(93)90076-a. [DOI] [PubMed] [Google Scholar]
- 17.Hornung RL, Longo DL, Gowda VL, Kwak LW. Induction of a CD8+ cytotoxic T lymphocyte response to soluble antigen given together with a novel muramyl dipeptide adjuvant, N-acetyl-d-glucosaminyl-(beta 1–4) -N-acetylmuramyl-l-alanyl-d-isoglutamine (GMDP) Ther Immunol. 1995;2:7–14. [PubMed] [Google Scholar]
- 18.Ke Y, Li Y, Kapp JA. Ovalbumin injected with complete Freund's adjuvant stimulates cytolytic responses. Eur J Immunol. 1995;25:549–53. doi: 10.1002/eji.1830250237. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi T, Kunisawa J, Hayashi A, et al. Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochem Biophys Res Commun. 1997;240:793–7. doi: 10.1006/bbrc.1997.7749. 10.1006/bbrc.1997.7749. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh NA, Rajananthanan P, Attard GS, Morrow WJ. Generation of antigen specific CD8+ cytotoxic T cells following immunization with soluble protein formulated with novel glycoside adjuvants. Vaccine. 1999;17:2974–82. doi: 10.1016/s0264-410x(99)00173-5. 10.1016/s0264-410x(99)00173-5. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi H, Takeshita T, Morein B, Putney S, Germain RN, Berzofsky JA. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990;344:873–5. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- 22.Dillon SB, Demuth SG, Schneider MA, et al. Induction of protective class I MHC-restricted CTL in mice by a recombinant influenza vaccine in aluminium hydroxide adjuvant. Vaccine. 1992;10:309–18. doi: 10.1016/0264-410x(92)90369-u. [DOI] [PubMed] [Google Scholar]
- 23.Raychaudhuri S, Morrow WJ. Can soluble antigens induce CD8+ cytotoxic T-cell responses? A paradox revisited. Immunol Today. 1993;14:344–8. doi: 10.1016/0167-5699(93)90233-B. [DOI] [PubMed] [Google Scholar]
- 24.Tan P, Visser E, Prestidge R, Watson J, Hiyama J, Skinner M, Scott L. Compounds and methods for treatment and diagnosis of mycobacterial infections. US Patent. 1999;5(985):287. [Google Scholar]
- 25.Skinner MA, Yuan S-N, Kim W-M, Watson JD, Tan PLJ. Mycobacterium vaccae as a vaccine and therapeutic agent for tuberculosis: role of natural killer cells. In: Griffin F, Lisle G, editors. Tuberculosis in Wildlife and Domestic Animals. Dunedin: University of Otago Press; 1995. pp. 123–5. [Google Scholar]
- 26.Ulmer JB, Sadoff JC, Liu MA. DNA vaccines. Curr Opin Immunol. 1996;8:531–6. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 27.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–51. doi: 10.1073/pnas.94.24.13146. 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudbach JA, Johnson DA, Ulrich JT. Ribi Adjuvants: Chemistry, biology and utility in vaccines for human and veterinary medicine. In: Stewart-Tull DES, editor. The Theory and Practical Application of Adjuvants. Chichester, UK: John Wiley & Sons Ltd; 1995. pp. 287–313. [Google Scholar]
- 29.Falk LA, Goldenthal KL, Esparza J, et al. Recombinant bacillus Calmette-Guerin as a potential vector for preventive HIV type 1 vaccines. AIDS Res Hum Retroviruses. 2000;16:91–8. doi: 10.1089/088922200309421. 10.1089/088922200309421. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM, Malin AS, Lukey PT, Atkinson SE, Content J, Huygen K, Dockrell HM. Characterization of human Mycobacterium bovis bacille Calmette-Guerin-reactive CD8+ T cells. Infect Immun. 1999;67:5223–30. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–22. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciupitu AM, Petersson M, O'Donnell CL, Williams K, Jindal S, Kiessling R, Welsh RM. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J Exp Med. 1998;187:685–91. doi: 10.1084/jem.187.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schirmbeck R, Melber K, Reimann J. Adjuvants that enhance priming of cytotoxic T cells to a Kb-restricted epitope processed from exogenous but not endogenous hepatitis B surface antigen. Int Immunol. 1999;11:1093–102. doi: 10.1093/intimm/11.7.1093. 10.1093/intimm/11.7.1093. [DOI] [PubMed] [Google Scholar]
- 34.Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein HSP60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- 35.Asea A, Kraeft S-K, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nature Med. 2000;6:435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 36.O'Neill LAJ, Dinarello CA. The IL-1 receptor/toll-like receptor suerfamily: crucial receptors for inflammation and host defence. Immunol Today. 2000;21:206–9. doi: 10.1016/s0167-5699(00)01611-x. 10.1016/s0167-5699(00)01611-x. [DOI] [PubMed] [Google Scholar]
- 37.Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. The CD14 ligands liparabinomannan and lipopolysaccharide differ in their requirement for toll-like receptors. J Immunol. 1999;163:6748–55. [PubMed] [Google Scholar]
- 38.Porgador A, Staats HF, Faiola B, Gilboa E, Palker T. J. Intranasal immunization with CTL epitope peptides from HIV-1 or ovalbumin and the mucosal adjuvant cholera toxin induces peptide-specific CTLs and protection against tumor development in vivo. J Immunol. 1997;158:834–41. [PubMed] [Google Scholar]
- 39.Abou-Zeid C, Gares MP, Inwald J, et al. Induction of a Type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997;65:1856–62. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang CC, Rook GAW. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998;93:307–13. doi: 10.1046/j.1365-2567.1998.00432.x. 10.1046/j.1365-2567.1998.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Reyn CF, Arbeit RD, Yeaman G, Waddell RD, Marsh BJ, Morin P, Modlin JF, Remold HG. Immunization of healthy adult subjects in the United States with inactivated Mycobacterium vaccae administered in a three-dose series. Clin Infect Dis. 1997;24:843–8. doi: 10.1093/clinids/24.5.843. [DOI] [PubMed] [Google Scholar]
- 42.Hrouda D, Baban B, Dunsmuir WD, Kirby RS, Dalgleish AG. Immunotherapy of advanced prostate cancer: a phase I/II trial using Mycobacterium vaccae (SRL172) Br J Urol. 1998;82:568–73. doi: 10.1046/j.1464-410x.1998.00803.x. [DOI] [PubMed] [Google Scholar]
- 43.Balagon MV, Walsh DS, Tan PL, et al. Improvement in psoriasis after intradermal administration of heat-killed Mycobacterium vaccae. Int J Dermatol. 2000;39:51–8. doi: 10.1046/j.1365-4362.2000.00862.x. 10.1046/j.1365-4362.2000.00862.x. [DOI] [PubMed] [Google Scholar]