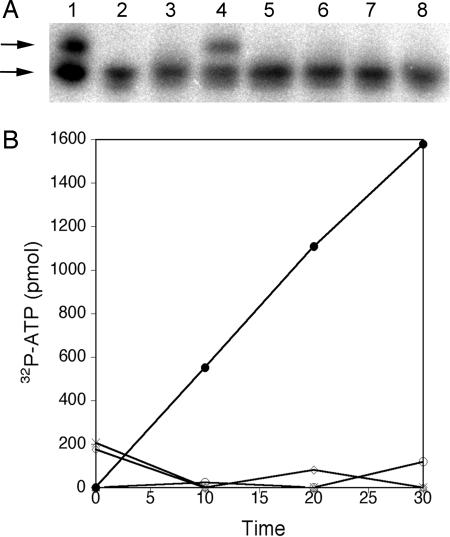
Fig. 3.
Cells bearing pylB, pylC, and pylD produce pyrrolysine detectable by in vitro assays using pyrrolysyl-tRNA synthetase. (A) The direct aminoacylation of tRNAPyl with pyrrolysine present in cell extracts by PylS. Charged and uncharged tRNA species in the isolated cellular pool of tRNAPyl were separated in an acid-urea polyacrylamide gel that was subsequently electroblotted. The blot was then probed with 32P-labeled oligodeoxynucleotide complementary to tRNAPyl and analyzed by phosphorimager. Lane 1 was loaded with cellular tRNA as isolated (9) and has both charged (upper arrow) and uncharged (lower arrow) tRNAPyl, whereas lane 2 is the cellular tRNA pool following deacylation at pH 9 for 30 min and shows only uncharged tRNAPyl. Aminoacylation of tRNAPyl by PylS with pyrrolysine was then tested in 25-μl reactions that contained: 3.2 μM PylS, 50 mM KCl, 1 mM MgCl2, 5 mM ATP, 0.5 mM DTT, 8 μg of M. acetivorans deacylated cellular tRNA, and the metabolite pool from the indicated E. coli strains in 10 mM Hepes buffer, pH 7.2. After incubation for 40 min at 37°C, aminoacylation was tested as above in reactions that also contained the following: lane 3, no metabolite pool; or the pool from, lane 4, pK13 bearing pylB, pylC, and pylD; 5, pK14 bearing pylB and pylD; 6, pK15 bearing pylC and pylD; 7, pK16 bearing pylB and pylC; and 8, pACYCDuet-1 bearing no pyl gene. (B) Cellular amino acid pools were tested for activity in the pyrophosphate:ATP exchange assay mediated by PylS in the presence of pyrrolysine. Exchange of 32P-pyrophosphate into ATP was monitored in 100-μl reactions containing 5.5 μM PylS, 10 mM MgCl2, 25 mM KCl, 1 mM potassium fluoride, 4 mM DTT, 2 mM ATP, and 2 mM 32P-PPi (12 dpm/pmol) in 20 mM Hepes-KOH (pH 7.2) and incubated at 37°C. Aliquots were removed at the time points indicated, and the amount of radiolabel bound to acid-washed activated charcoal was quantified to estimate the amount of 32ATP formed. Shown are illustrated results from averaged duplicate reactions that were supplemented with the extracted amino acid pool from pK13 (●), pK14 (X), pK15 (△), pK16 (◇), or pACYCDuet-1 (○).