Figure 2.
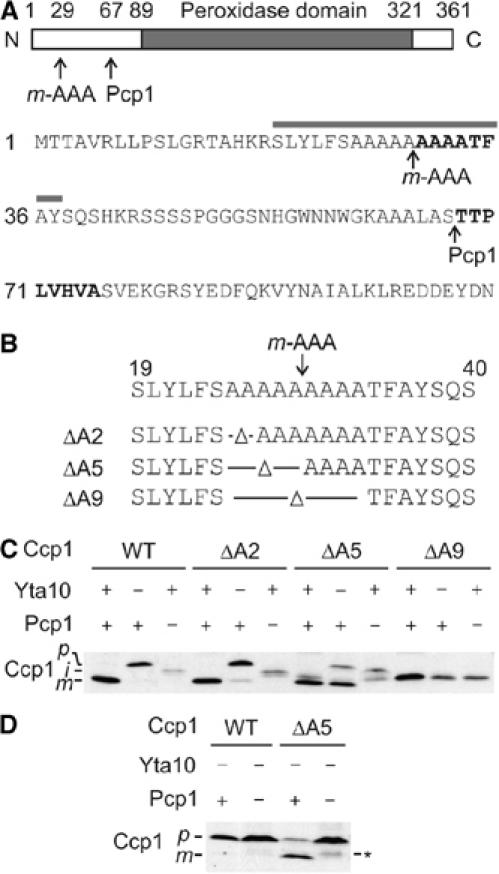
An alanine stretch within the Ccp1 presequence determines the m-AAA dependence of rhomboid cleavage. (A) Upper panel: Domain structure of Ccp1. Processing sites of the m-AAA protease and Pcp1 within Ccp1 and the peroxidase domain of Ccp1 are indicated. Lower panel: Amino-acid sequence of the N-terminal region of the Ccp1 precursor protein. N-terminal amino-acid residues determined by Edman sequencing of intermediate and mature forms of Ccp1 are shown in bold. The grey bar indicates the 19-amino-acid window used in Figure 3D. (B) Schematic representation of Ccp1 mutants lacking two (ΔA2), five (ΔA5) or nine (ΔA9) alanine residues surrounding the processing site of the m-AAA protease. (C) In vivo processing of Ccp1 variants harbouring mutations in the alanine stretch. Lysates of Δccp1 (YTT266), Δyta10Δccp1 (YTT269) or Δpcp1Δccp1 (YTT271) cells expressing Ccp1 or mutant variants were analysed by SDS–PAGE and Western blotting using Ccp1-specific antiserum. p, precursor form; i, intermediate form; m, mature form. (D) In vivo processing of Ccp1ΔA5. Lysates of Δyta10Δpcp1Δccp1 (YTT273) cells expressing Ccp1 or Ccp1ΔA5 (ΔA5) were analysed as in (C). p, precursor form; m, mature form. The asterisk indicates a form of Ccp1ΔA5 generated by an unknown peptidase.
