Figure 3.
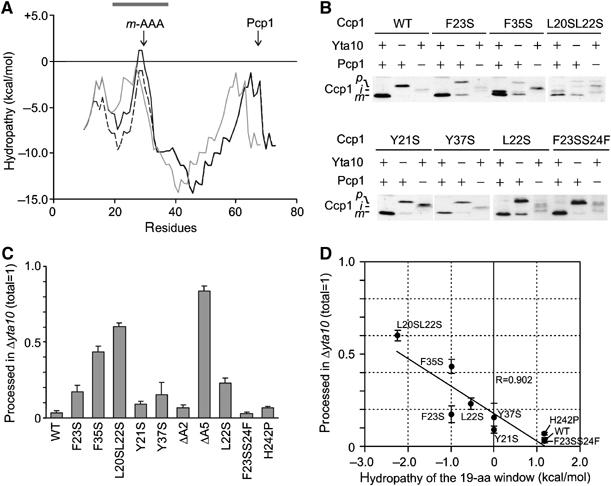
The hydrophobicity of the Ccp1 sorting signal determines the m-AAA protease dependence of Ccp1 processing. (A) Hydropathy plot of the N-terminal 80 amino acids of Ccp1 (black line), Ccp1ΔA5 (grey line) and Ccp1F23S (dotted line) according to MPEx. The grey bar indicates the 19-amino-acid window used in (D). (B) In vivo processing of Ccp1 (WT) and mutant variants. Samples were analysed as in Figure 1C. (C) Quantification of m-AAA protease-independent processing of Ccp1 mutants. Precursor and mature forms of Ccp1 or their variants were detected in Δyta10Δccp1 (YTT269) cells using an infrared scanner (Odyssey). (D) Correlation of processing efficiencies in Δyta10Δccp1 with the hydrophobicity of the sorting signal. Amounts of mature Ccp1 (total was set to 1) were plotted against the hydropathy of the 19-amino-acid window (centre=A29) of Ccp1 variants. Deletion mutants in the sorting signal were not considered for this analysis as the deletion causes a shift of the window.
