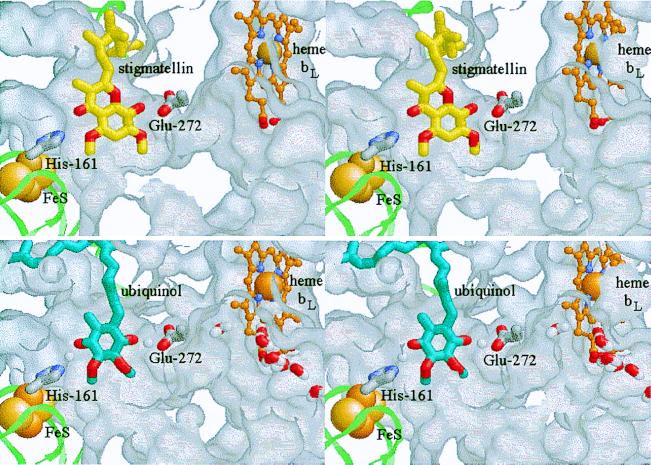
Figure 1.
Stigmatellin binding as a model for the enzyme–substrate complex. (Stereo pairs.) The ISP is shown as a pale green ribbon, with the 2Fe–2S cluster as space-filling spheres. cyt b is represented by the exterior surface of the protein, mapped with a 1.4-Å probe. The protein has been cut away to reveal the Qo-site volume. The side chains of His-161 (ISP) and Glu-272 (cyt b) are shown as Corey–Pauling–Koltun (CPK) colored tube models, and heme bL as a ball-and-stick model, with the Fe atom space-filling and C atoms in orange. (Upper) The binding of stigmatellin (C atoms yellow, O atoms red; from PDB 2bcc). (Lower) The enzyme–substrate complex: ubiquinol is shown with C atoms blue, O atoms red. A putative water chain (bottom right) occupies a channel in cyt b leading from the external aqueous phase to the heme bL binding pocket and the Qo pocket. See Experimental Procedures for model building and the text for discussion. These and the native and myxothiazol- and quinone-containing structures are available as supplementary material for interactive viewing through a Chime tutorial at www.pnas.org.