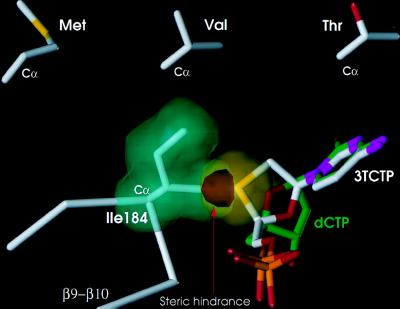
Figure 4.
Schematic illustration of proposed steric conflict between 3TCTP and 184I. The van der Waals volume of the side chain of I184 (green) is shown to overlap (red) with the sulfur of the β-l-oxathiolane ring of the incoming 3TCTP (yellow). A dCTP molecule (green) is shown superimposed on 3TCTP (white) in a way that maintains the same base pairing with a modeled template strand and proximity to the 3′-OH, but without steric conflict with I184. The model predicts similar steric hindrance with the two other β-branched amino acids (Val and Thr). The side chains of these amino acids are shown in an orientation similar to the I184 observed in our structure. This model is supported by the reported resistance of M184V and M184T RT to 3TCTP. An analogous model can explain resistance of analogous mutants of HBV RT to 3TCTP.