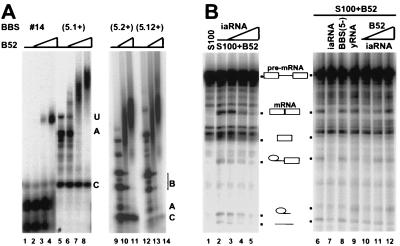
Figure 2.
In vitro characterization of the iaRNA. (A) Comparison of the avidity of the pentavalent iaRNA to the affinity of a monovalent aptamer. A template containing a single pentavalent monomeric unit, BBS (5.1+), was transcribed by T7 RNA polymerase to produce the 32P-labeled RNA probe. Its avidity to B52 was compared with the affinity of the primary aptamer BBS #14 in a band shift assay. The adjacent lanes in each set have a 10-fold difference in B52 concentration, the lowest being 5 nM. The molar ratio of pentamer to monomer used in different sets was 1:5. The uncut transcript of BBS (5.1), Fragment A, and Fragment C in the left gel are indicated by U, A, and C, respectively. RNA transcribed from longer templates was run on a separate gel (Right). The B/A and B/C ratio increased when the template became longer as indicated. (B) Inhibition of B52’s splicing activity with the iaRNA. Labeled ftz pre-mRNA was used as the substrate in a splicing assay in which B52 complemented a splicing-deficient S100 extract from Kc cells. This activity was challenged by adding the iaRNA (Fragment B), its antisense RNA [BBS (5−)], or purified yeast RNA. The iaRNA-suppressed activity was restored subsequently by additional B52 protein. Splicing products and intermediates of ftz are indicated schematically between the two gels. Exons are represented by boxes, introns by lines.